
The incidents that might result from the use of medical devices are followed up by materiovigilance. It helps to identify the adverse events associated with the use of medical devices. Haemovigilance primarily aims to assure the surveillance of blood transfusion, from blood collection, storage and further steps involved. A collection of data in haemovigilance helps in framing vital changes in the whole blood transfusion process. To overcome problems related to medical devices and blood transfusion and in order to improve the standards of the healthcare system for the betterment of the patients, various software are introduced so that appropriate corrective actions can be taken in the field.
The healthcare system in India provides great discrepancy in quality. To maintain the quality of healthcare system and to protect the health and safety of patients, comprehensive vigilance is required. Materiovigilance and haemovigilance programs have evolved to reduce the likelihood of recurrence of the harmful incidents, thereby improving the quality of health products and health services. The need for this vigilance was felt due to malfunctioning of medical devices (instruments, apparatuses, and materials etc. which are intended for medical purpose and are an integral part of the health system to health providers and patients/ consumers). Thus, materiovigilance is a system to monitor the safety of medical devices in the country.
Haemovigilance aims to improve the overall safety of blood transfusion by detecting and analysing all untoward effects of blood transfusion, to correct their cause and to prevent recurrence. Haemovigilance has evolved from pharmacovigilance. Haemovigilance as defined by Faber is “a set of surveillance procedures covering the whole transfusion chain (from the donation of blood and its components to the follow-up of recipients of transfusion), intended to collect and assess information on unexpected or undesirable effects resulting from the therapeutic use of labile blood products and to prevent the occurrence or recurrence of such incidents". Materiovigilance is related to haemovigilance in relation to the patient’s safety. If material vigilance is not so strict in terms of quality assurance, several pyrogens, toxic chemicals or hazardous materials acquired by gaseous sterilisation or fibres which may release from poor raw materials or techniques might be entrapped into the cardiovascular system. On the other hand, if whole blood is not preserved properly, it may lead to the variety of problems associated with the safety of blood transfusion.
However, constructive collaborations of industries, blood organisations, blood centres, clinical segment, and authorities in coordinated ways help in smooth functioning of materiovigilance and haemovigilance. There are many problems and difficulties at various stages of materiovigilance and haemovigilance to which the solutions need to be sought for the future. Various elements will be considered for the meticulous operation of the materiovigilance and haemovigilance such as the introduction of various softwares etc. In theory, the different vigilance systems are defined, but not implemented practically.
Additionally, much focus has to be given for the organ vigilance since very limited information is available about the serious reactions and adverse effects related to the donation and transportation of organs. A national surveillance and vigilance system is essential for monitoring the donation and transportation of organs in order to improve the quality systems in these sectors. Therefore, the objective of our review work is to highlight the significance of materiovigilance, haemovigilance and organovigilance that have been proved to be quite vital components for quality management in healthcare of human population.
MvPI was launched by DCG (I) on 6th July, 2015 at Indian Pharmacopoeia Commission (IPC) Ghaziabad. Indian Pharmacopoeia Commission functions as a National Coordination Centre (NCC) for MvPI. The details can be obtained from the website.
Presently only some devices are included for the materiovigilance such as cardiac stent, drug eluting stents, catheters, intraocular lenses, bone cement, heart valves, scalp vein sets, orthopedic implants and disposal perfusion sets.
• Analysing the benefit-risk ratio of various medical devices
• The information on the safety of medical devices should be generated on the basis of the evidence
• MvPI should support Central Drugs Standard Control Organisation in the decision-making process on the use of medical devices
• To minimise the risk, the safety information on the use of medical devices should be communicated to various stakeholders
• Proper functioning of MvPI would lead to emergence of it as a national centre of excellence for materiovigilance activities which can be further collaborated with other healthcare organisations for the exchange of information and data management.
MDAEs are meant to ensure and generate awareness among all the professionals involved in the healthcare segment regarding the importance of MDAEs reporting in the country and to keep a check on the benefit-risk profile of the medical devices so that independent and evidence based recommendations are generated for the sake of the safety of medical devices. It ensures further communication of the findings to all the concerned key stakeholders in the country including the stakeholders of the Pharmacovigilance Programme of India (PvPI).
All the serious or not serious, known and unknown types of suspected MDAEs can be reported. The MDAEs reporting form should include all adverse events associated with the use of medical devices, the description of the incident, description of the device (deficiency or malfunction), and illumination of hazards linked with the device including the associated menace of the patient.
All the healthcare professionals involved including clinicians, pharmacists, doctors, nurses and patients/consumers can report MDAEs to Sree Chitra Tirunal Institute of Medical Science and Technology (SCTIMST) or National Collaboration Centre (NCC). Duly filled MDAEs Reporting Form can be sent to SCTIMST, NCC, MvPI, Biomedical Technology Wing, Poojappura, Thrivananthapuram-695012, Kerala, India or can directly email the duly filled form to mvpi@sctimst.ac.in.
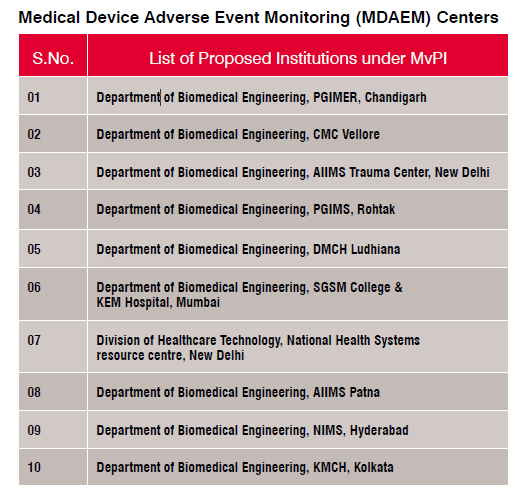
MDAEs Reporting Form can be downloaded from the website of IPC (www.ipc.gov.in) to report an adverse event associated with medical devices. MDAEs can also be reported via PvPI helpline number (1800 180 3024) on weekdays from 9:00 am to 5:30 pm.
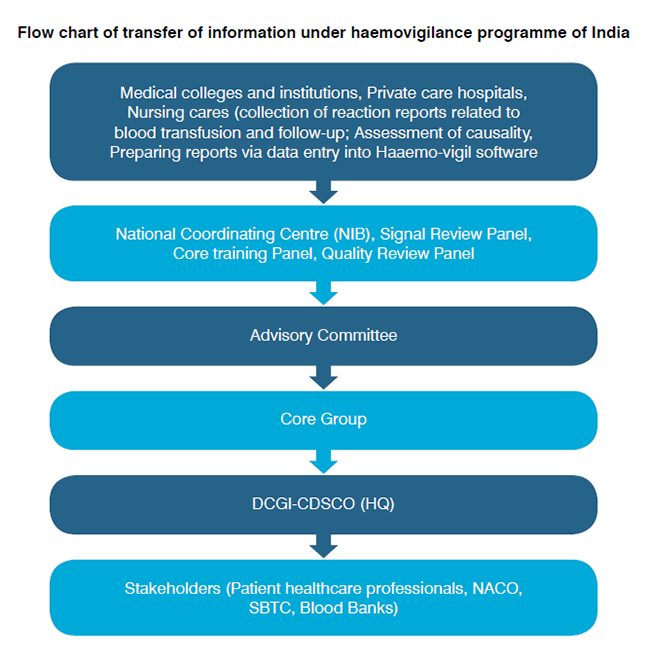
The haemovigilance system should cover process throughout the entire transfusion chain, from blood donation, processing, and transfusion to patients for the monitoring, reporting, and investigation of adverse events and reactions and near misses related to blood transfusion. The blood transfusion service should be well coordinated in each and every stage starting from the hospital, blood banks/ blood donors, clinical staff and transfusion laboratories, hospital transfusion committees, related regulatory agency and ultimately the national health authorities.
The blood transfusion may often result in the detrimental manner in the donors and the recipients:
1. Local reactions occur predominantly because of problems related to venous access: The incorrect placement of the needle during the venipuncture may lead to haematomas due to extravasations from the veins. Pain, hyperemia, and swelling may develop at the site of extravasation. Other local events include pain due to slight trauma to the subcutaneous nerve endings. Local phlebitis and thrombophlebitis are more serious complications than the foregoing but very rare.
2. The systemic reactions in contrast to the local reactions can be divided into mild or severe type. In most cases, they are vasovagal reactions that can be triggered by the pain of the venipuncture. Apheres is procedures may lead to systemic reactions which require the use of anticoagulants (such as acid-citrate-dextrose) for the collection of blood component often causing hypocalcemia, because of chelation.
3. Recipient haemovigilance: The International scientific society has laid down criteria for ‘severity' (of an adverse event)and ‘imputability' (that is, the likelihood be attributed to the blood component transfused) of transfusion reactions. The reactions due to transfusion in a recipient are classified as acute (within 24 hours) or delayed (after hours of transfusion) reaction.
4. Haemovigilance related to blood donors: All the reports of unexpected adverse events in whole blood and component donors should be included in the donor haemovigilance and action must be taken. The recipient might be at risk due to the events such as adverse reactions or complications arising from the donation, selection, and management of donors, which in turn may harm the donor or affect the quality of the product. An active initiative from the International Scientific Society and European Heart Network has been taken to propose various classification and a set of definitions of complications related to blood donation.
Mild to serious problems can be encountered if the process of blood transfusion goes wrong at any step. Unsafe blood transfusion procedures may result in various allergic reactions, diseases of infectious and viral nature, acute immune hemolytic diseases, sepsis from bacterial contamination, delayed hemolytic reaction, graftversus-host disease, trauma, hepatitis B and C, human immunodeficiency virus.
NCC National Coordinating Centre issues Transfusion reaction reporting form to ensure risk minimisation, to put a check on the issues associated with blood transfusion process and to report adverse reactions and adverse events resulting from blood transfusion such as allergic or immunological reactions which arise from the blood transfusion.
1. The legal and other mandatory frameworks should be in place according to the country
2. The common definitions must be agreed
3. Standardised method of reporting should be used throughout
4. To ensure proper functioning of the chain, continuous budgeting and financing should be guaranteed
5. Central evaluation site must be established with the culture of professionalism
6. Hospital Transfusion Committees should work efficiently
7. The mechanisms for corrective and preventive actions must be introduced
The outline of this process is similar at each level where haemovigilance and materiovigilance are related as it applies to industries manufacturing disposables such as syringe, gauze, tubes and related items, blood centres, wards, hospitals, competent authorities, manufacturers, etc. The main participants involved in this process are manufacturers, physicians, pharmacists, nurses, medical technicians, etc. Communication channels between the different levels must be established and predefined. For adequate working of the whole system for the betterment of the public health, it becomes vital that close and constructive cooperation is established between the different participants of the different levels, at different sites, and with many different people involved. It becomes crucial to settle the organisational aspects, to expound the corresponding responsibilities and instructions, to increase responsiveness with clear written procedures in a ‘no blame environment’, to offer adequate and continuous training.
The blood centres and hospitals served by the manufacturers of materials, disposables, reagents, equipments, etc. hence, it becomes vital to regularise
the requirements (community, national) and the post-marketing procedures of companies as a sturdy tool for the collection and compilation of data directly and indirectly related to haemovigilance. Acceleration of the input and involvement of the industry into haemovigilance will be beneficial in public interest.
Blood centers/blood banks are the consumers of the materials such as disposables, reagents, equipments, etc., manufactured by the industry. At the same time, they are also the producers of labile blood components of all types, as well as the providers of transfusionrelated services. In this way, they play a vital role in haemovigilance, serving as users on one hand and producers on the other hand.
The experts involved in the clinical segment are in a position to detect and report events, side effects, incidents, reactions, accidents, errors, nearmisses, etc. Being at the frontline, physicians and paramedics play a crucial role and should readily collaborate in a constructive and coordinated way for the fulfilment of its overall aim, for example, to increase safety and quality of the blood transfusion process in the best interest of those patients who are in need of labile blood.
There is still a deficit in relation to haemovigilance and materiovigilance when it comes to common definitions, terminology, standardised reporting and uniform matrix. Some of the most ubiquitous problem such as organisation problems due to lack of proper funding, lack of professionalism, unclear mandates, indeterminate responsibilities, low sensitivity, inadequate training, hesitation to move forward by implementing strong actions, lack of harmonisation of data collected from different sources, as a result of which exiguous results are generated, the final reports turns out to be mutable and less dependable.
Empowerment and enlightenment of knowledge and the right skills along with computer-friendly attitude had lead to the strengthening of the linkage of organisation with consumer/patient. The smooth working of organisations depends on the right type of people who have necessary perspective and understanding of function, products, organisation and a desire to be customer responsive.
The seamless flow of work allows employees in the organisation to route consumer's communication through a virtual folder that combines documents, voice messages, e-mails, faxes, and so forth, to be delivered to the nearest consumer service points at the right time to ensure single point resolution of any consumer complaint or enquiry.
Two software: (1)Haemo-vigil for recipient Haemovigilance and (2) Donor-vigil software for donor Haemovigilance was utilised as sample tracking web-application by National Institute of Biologicals, Ministry of Health and family welfare, the Government of India. The response and use of software depend on knowledge, integrity, and truthfulness of patent, otherwise the data obtained could be misleading in interpretation.
Moreover, this software is used by different end users. There are serious inconsistency problems in the development of programs to access such data. The integrity (i.e. the accuracy and completeness) of the data was in question because there was no control over their use and maintenance by authorised end use.
Time is an indicator of opportunity. Now the internet is a powerful tool for diffusing information and knowledge. Internet communication can also have some negative impact such as the early version of Pentium chip in the 1990s contained a bug that made some mathematical expression and calculation go wrong. This was first discovered by Prof. Thomas Nicely, professor of mathematics, Lynchburg Collage. Thus mishandling and misuse of internet facility can have a negative impact on ‘Haemo-vigil’ and ‘Donorvigil’ software.
Manufacturing organisations are concentrating on improvement by laying stress on productivity, quality, energy conservation, safety and also on environmental protection. They have been able to improve operational performance through significant improvement in its TPM, while the regulatory authority could not improve their operational performance to identify and prevent occurrence or recurrence of transfusion-related unwanted events to increase the safety, efficacy, and efficiency of blood transfusion, covering all activities of transfusion chain from donor to recipient.
Through different projects, it provides healthcare activities for HIV positive patient's education and support for their children with challenges a hospice for dying destitute, basic education for children in rural areas, and support to government relief measures in natural calamities.
Data mining acts as an essential tool in the extraction of hidden predictive information from large databases. It is a robust technology with great potential to help the regulatory organisations focus on the most important information. Data mining tools predict future trends and behaviours, knowledge-driven information and even decisions. Materiovigilance and haemovigilance program of India may collect and refine massive quantities of data. Data mining technique could be implemented rapidly on existing software and hardware platform to enhance the value of existing information resources and can be integrated with new systems as they are brought online.

The regulatory authority can analyse its recent adverse drug reactions and their results to improve targeting of high-value physicians and determine which vigilant measures will have the greatest impact transfusion. The data needs to include NGOs’ activity as well as information about the local healthcare systems. The ongoing dynamic analysis of the data warehouse allows best practices from throughout the regulatory organisations to be applied in specific vigilance activity. Data mining algorithms embody techniques and advanced statistical and computational methods can interpret data from diverse sources into a meaningful signal to benefit patient safety.
SWOT analysis (strengths, weakness, opportunity, and threats) is to be used to evaluate the impact of both materioviglilance and haemovigilance. The MvPI and HPV's strengths are its core competencies and resources in which it functions. Weakness is areas of unexplored performances. Opportunities are the potential for new or innovative breakthroughs that might greatly expand its prospect. Threats are potential for disruptive new technologies both in MvPI and HvPI.

The challenge is to understand not just the internet feedback but even the sociology of human networks. The reliability, responsiveness, and assurance of feedback on any vigilance are also important. An Adverse Event Following Immunisation (AEFI) and haemovigilance are important divisions of PvPI to which ADRs following immunisation and transfusion of blood / blood products are to be reported and recorded. One important aspect of haemovigilance and AEFI is that it is difficult to persuade the blood bank staff and clinicians to report cases of ADR due to the fear of legal and regulatory repercussions.
Health records in electronic form: An integrated collection of data extracted from operational, historical and external databases at multiple locations such as hospitals, physicians clinic, health management organisation (HMO).An expert system (ES) is a knowledgebased information system that uses its knowledge about a specific (materiovigilance, haemovigilance), complex application area to act as an expert consultant to end users. We know a variety of manufacturing information systems available and many of them web-enabled, which are used to support Computer-Integrated Manufacturing (CIM). It may be suggested that the pharmacovigilance, materiovigilance or haemovigilance may be designed in such a way that computerintegrated haemovigilance, materiovigilance or pharmacovigilance to get the highest quality of vigilance for the benefit of society.
Nowadays, the virtual reality is applied widely in different field such as computer-aided design (CAD), medical diagnostics and treatment, scientific experimentation in many physical and biological sciences. It may also be applied to MvPI, HvPI's.
DISCUSSION
The present scenario of healthcare system in India highlights the need for strict vigilance to safeguard the health and safety of patients. The patient safety requires continual enhancement of quality and safety of related materials and the blood products for the transfusion process by monitoring and safeguarding the adverse event associated with the use of materials and blood products. In this regard, materiovigilance and haemovigilance have proved to be essential components of quality management in healthcare fraternity. They have minimised the risk of any unwanted incidences, thereby improving the healthcare products and services. The materovigilance aids in combating the harmful incidents occurring due to medical devices, while haemovigilance aims to monitor any untoward reactions occurring during blood transfusion and to prevent their recurrence. Only some medical devices are enlisted as per MvPI, which includes cardiac stent, drug eluting-stents, catheters, intraocular lenses, bone cement, heart valves, scalp vein sets, orthopaedic implants, disposal perfusion sets, but several other devices are to be undertaken for surveillance for the benefit of patients such as glaucoma valve, eyeball (Brachy therapy radioactive plaque), retinal band and buckle, pacemaker, etc.[10]. Moreover, organovigilance may be considered as it is the latest echnology driven so as organ transplantation hazards could be avoided and the safety of recipients of organs could be maintained.
CONCLUSION
A streamlined mechanism along with good coordination using standardised tools at every level is required for proper functioning of materiovigilance and haemovigilance. The need to exercise prudence is indispensable starting from materials of the products, packaging, labelling, instructions, selection of the donors, collection, and handling, storage of the blood and blood products, and various stages of transfusion to monitor the adverse reactions if any. A functional hospital transfusion committee can act as a backbone for this by developing policies for transfusion practices, appropriate documentation, reporting and investigation of transfusion reactions. Following the draft recommendation on haemovigilance system by World Health Organization (WHO) may help in developing an efficient system in developing countries. Thus, considering patient safety as prime goal, materiovigilance and haemovigilance should be monitored by the national authority.
REFERENCES
1. Cheikh A, El Wartiti A, Enneffah W, Badsi N, Ajaja M, Al Baroudi S, et al. PS-036 The place of materiovigilance as tool of traceability in the quality management system (QMS) in hospital pharmacy. 2015;A150.
2. Faber DJ. Haemovigilance around the world. Vox Sang 2002;83:71-76.
3. Faber JC. Hemovigilance: Definition and overview of current hemovigilance systems. TransfusAlternTransfus Med 2003;5:237-245.
4. Boparai JK, Singh S. Hemovigilance: A new beginning in India. Int J App Basic Med Res 2015;5:200-202.
5. Dieffaga T, Sanogo M, Maiga S. P360: Materiovigilance and improvement of the maintenance of the biomedical equipment by the implementation of strategies for the use of equipment: case study of the hospital Gabriel Touré of Mali. Antimicrob Resist Infect Control 2013;2:P360.
6. Mehakovic E. Safety and Vigilance in Organ Donation for Transplantation. Transplantation 2017;101:S97.
7. MateriovigilanceProgramme of India (MvPI). Available from: http://ipc.nic.in/writereaddata/mainlinkFile/File572.pdf.
8. Handbook of Regulatory Guidelines for Medical Devices & in vitro Diagnostic Kits; 2016. Available from: http://cdsaindia.in/sites/default/files/MedicalDeviceHandbook_CDSA_2016.pdf.
9. Kumar P, Kalaiselvan V, Kaur I, Thota P, Singh GN. MateriovigilanceProgramme of India (MVPI): a step towards patient safety for medical devices. European J Biomed Pharm Sci 2016;3:497-501.
10. De Vries RR, Faber JC, editors. Hemovigilance: an effective tool for improving transfusion safety. John Wiley & Sons; 2012.
11. Philip J, Sarkar RS, Pathak A. Adverse events associated with apheresis procedures: Incidence and relative frequency. Asian J TransfusSci 2013;7:37-41.
12. Crocco A, D’Elia D. Adverse reactions during voluntary donation of blood and/or blood components. A statistical-epidemiological study. Blood Transfus 2007;5:143-152.
13. Sen S, Gupta P, Sinha S, Bhambani P. Haemovigilance and transfusion safety: A Review. Sch J App Med Sci 2014;2:85-90.
14. Sharma G, Sadhna D, Kaushik KN. Haemovigilance: a system to improve safety in blood transfusion process. World J Pharm PharmSci 2014;3:1889-1898.
15. Transfusion Reaction Reporting Form (TRRF) for Blood & Blood Products. Indian Pharmacopoeia Commission – National Institute of Biologicals Ministry of Health & Family Welfare – Govt. of India. Haemovigilance (PharmacovigilanceProgramme of India). Available from: http:// http://www.ijp-online.com/documents/Transfusion.pdf.
16. Faber JC. Worldwide overview of existing haemovigilance systems. TransfusApherSci 2004;31:99-110.
17. Jain A, Kaur R. Hemovigilance and blood safety. Asian J TransfusSci 2012;6:137-138.
18. Stainsby D, Faber JC, Jørgensen J. Overview of hemovigilance. Rossi's Principles of Transfusion Medicine, Fourth Edition; 2009:681-698.
19. Mukherjee S, Maiti R. Haemovigilance: A Current Update in Indian Perspective. J ClinDiagn Res 2016;10:EE05-EE09.
20. McKone KE, Weiss EN. Total Productive Maintenance (TPM). Innovations in Competitive Manufacturing, Springer US 2000; p. 187-197.
21. Venkatesh J. An introduction to total productive maintenance. Plant Maintenance Resource Center 2007; 3-20.
22. Milley A. Healthcare and data mining. Health ManagTechnol 2000;21:44-47.
23. Milovic B, Milovic M. Prediction and Decision Making in Health Care using Data Mining. Int J Public Health Sci 2012;1:69-78.
24. Lindquist M, Ståhl M, Bate A, Edwards IR, Meyboom RH. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf 2000;23:533-542.
25. Van Wijngaarden JD, Scholten GR, van Wijk KP. Strategic analysis for health care organizations: the suitability of the SWOT?analysis. Int J Health Plann Manage 2012;27:34-49.
26. Suke SG, Kosta P, Negi H. Role of pharmacovigilance in India: An overview. Online J Public Health Inform 2015;7:e223.
27. Hogan WR, Wagner MM. Accuracy of data in computer-based patient records. J Am Med Inform Assoc 1997;4:342-355.
28. Detmer DE, Steen EB, Dick RS, editors. The computer-based patient record: an essential technology for health care. National Academies Press; 1997.
29. van den Burg PJ, Magnussen K. Ethical aspects of blood donors and the recipients of their blood. J Blood Transfus 2012;2012.