New treatment modalities such as immunotherapeutic strategies may help improve the currently poor prognosis and outcome of patients suffering from lung cancer. However, thus far, lung cancer has not been considered an immune-sensitive malignancy. Now, there is an increasing evidence that specific humeral and cellular anti-tumour immune responses can be evoked.
Lung cancer is the deadliest cancer in the world. In 2005, in the US alone, there were an estimated 163,510 deaths in patients suffering from lung cancer, including 15,000 to 20,000 “never smokers”1. These numbers clearly demonstrate that despite progress in the treatment of this disease over the past two decades, there are still few long-term survivors: only about 10% of all patients will ever be cured of this devastating disease2.
Given the modest effect and considerable toxicity of current standard treatment (surgery, chemotherapy, radiation therapy), there is clearly a need for novel treatment options. Currently, a wide variety of immunotherapeutic agents are being tested in lung cancer.
Here, we review strategies based on the humeral and cellular immune system that are already in clinical use or well progressed in early clinical trials. Because Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 80% of all lung cancers, the focus is mainly set on immunotherapy of NSCLC.
Antibody-based Immunotherapy
Today, at least 12 monoclonal antibodies (mAB) have received FDA approval and over 400 others are being tested in clinical trials3. In lung cancer, a primary focus was put on mABs targeting the Epidermal Growth Factor Receptor (EGFR) and the Vascular Endothelial Growth Factor (VEGF)4. The most advanced in development are the anti-EGFR mAB cetuximab (Erbitux®) and the anti-VEGF mAB bevacizumab (Avastin®).
Antibodies targeting growth factors
To block growth factors and their receptors seems an obvious strategy in fighting cancer because they are known to augment tumour cell proliferation and invasion and have been shown to be overexpressed in many solid malignancies where the overexpression has been associated with a more aggressive course of disease and poor survival5. C-erb B-1 and c-erb B-2 are the two growth receptor families that have been studied most extensively. C-erb B-1 is better known under the name HER1 or Epithelial Growth Factor Receptor (EGFR). HER2 is the more common name for c-erb B-2.
Anti-EGFR (anti-c-erb B-1) Monoclonal Antibodies
Cetuximab (Erbitux®), a chimeric human:murine form of the original mAB 225, has demonstrated safety and was well tolerated in early phase clinical trials, but low patient numbers currently do not allow for final assessment of its therapeutic efficacy in lung cancer. A 28% partial response rate and 17% of patients with stable disease were observed in a Phase II trial with combination of cetuximab and docetaxel which exceeds response rates usually seen with docetaxel alone6. In another phase II trial patients with recurrent or progressive NSCLC were treated with cetuximab after receiving at least one prior chemotherapy regimen7. The response rate for all patients (n = 66) was 4.5% and the stable disease rate was 30.3%.
The median time to progression for all patients was 2.3 months and median survival time was 8.9 months. The authors of this study concluded that, although the response rate with single-agent cetuximab in this heavily pretreated patient population with advanced NSCLC was only 4.5%, the disease control rates and overall survival seemed comparable to that of pemetrexed, docetaxel and erlotinib in similar groups of patients. More Phase I/II clinical trials have been conducted on the use of cetuximab in combination with systemic chemotherapy and / or radiation therapy confirming not only the low toxicity but also the clinical response rates8,9.
Grade 3 toxicities associated with the use of cetuximab were fatigue, infections and papulopustular rash. Development of the rash, usually located on the face and upper torso, has been related to a clinical response and has been suggested to potentially serve as a surrogate marker for cetuximab activity10. Other anti-EGFR mABs currently in development include panitumumab (ABX-EGF), matuzumab (EMD 72000), pertuzumab (2C4) and MDX21411. Early phase clinical trials with these agents in patients with lung cancer are currently ongoing with results pending.
Anti-HER2 (anti-c-erb B-2) Monoclonal Antibodies
Trastuzumab (Herceptin®), a humanised monoclonal antibody that targets the HER2 receptor, has been approved for metastatic breast cancer. However, to date, it failed to demonstrate clinical efficacy in patients suffering from lung cancer12,13. Only few authors suggest further investigation of trastuzumab in HER2-positive lung cancer14. In contrast, pertuzumab (2C4), a mAB designed to inhibit the dimerisation of HER2 with EGFR and other HER tyrosine kinases and, therefore, being independent of HER2 overexpression is currently under evaluation in NSCLC in early phase clinical trials11.
Monoclonal Antibodies against other growth factors
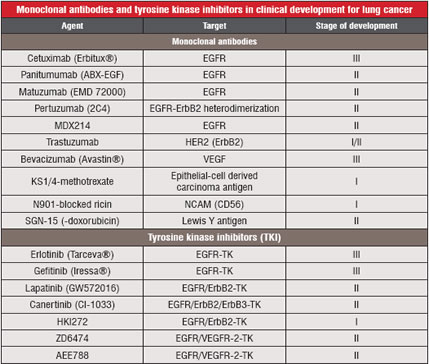 Table 1: Lists Antibodies and Tyrosine
Table 1: Lists Antibodies and Tyrosine
Kinase Inhibitors Currently under Clinical Investigation for Lung Cancer. Other factors relevant for tumour cell proliferation, such as the intercellular adhesion molecule-1 (ICAM-1)15, have been identified as targets for mABs. Of these, bevacizumab (Avastin®), which targets the Vascular Endothelial Growth Factor (VEGF) recently gained approval for the treatment of colorectal cancer. A phase II clinical trial using bevacizumab alone or in combination with chemotherapy in patients with metastatic NSCLC revealed promising results16. Other studies investigated the use of bevacizumab, for e.g., in combination with the EGFR-tyrosine kinase inhibitor erlotinib (Tarceva®) or as combination therapy with paclitaxel and carboplatin in the neoadjuvant setting17,18. In the largest trial evaluating bevacizumab, 878 patients with recurrent or advanced NSCLC (stages IIIB and IV) were included19. The median survival was 12.3 months in the group assigned to chemotherapy plus bevacizumab, as compared with 10.3 months in the chemotherapy-alone group. The median progression-free survival in the two groups was 6.2 and 4.5 months, respectively, with corresponding response rates of 35% and 15%. Rates of clinically significant bleeding were 4.4% and 0.7%, respectively.
Other Antibody-based Immunotherapeutic Approaches
In Small-Cell Lung Cancer (SCLC), an anti-idiotype vaccine targeting the ganglioside GD3 (BEC2) has been evaluated20. The European Organization for Research and Treatment of Cancer (EORTC) recently published data from a phase III trial using BEC2 in combination with induction chemoradiotherapy in limited stage SCLC21. A total of 515 patients were randomly assigned to receive five vaccinations of BEC2 (2.5 mg)/BCG vaccine or follow-up. There was no improvement in survival, progression-free survival, or quality of life in the vaccination arm. Among vaccinated patients, a trend toward prolonged survival was observed in those who developed a humeral response.
To increase their cytocidal potency, mABs are being linked to cytocidal agents, such as toxins, chemotherapeutic drugs or radionuclides. Approved for clinical use are, for e.g., gemtuzumab ozogamicin (Mylotarg®), which links the toxin calicheamicin to a CD33-specific antibody for use in the treatment of myelogenous leukemia and ibritumomab tiuxetan (Zevalin®), which links 90Y to a CD20-specific mAB. To date, the available data on comparable antibodies for the treatment of lung cancer is, however, very limited and very few reports on clinical applications are available22-24.
Therapeutic Lung Cancer Vaccines
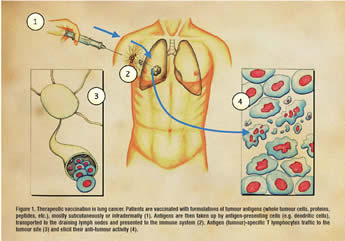 In contrast to the prophylactic vaccination against infectious disease or cancers associated with viral infection (cervical cancer, hepatocellular carcinoma), for cancer patients the only relevant vaccination strategy must be therapeutic. Generally speaking, cancer vaccines incorporate a source of tumour antigens combined with some type of “adjuvant” to make these tumour antigens visible to the immune system. Sources of tumour-associated antigens include whole autologous or allogeneic tumour cells, defined proteins, or specific peptide epitopes (see Figure 1). Most likely due to the heterogeneous histology of lung cancers, the relevant immunologically dominant antigens remain unknown. Therefore, the use of autologous tumour cells might be especially suitable for vaccination strategies in lung cancer, because no prior knowledge of specific tumour antigens is necessary and the induced immunity may not be confined to a single, specific antigen that could be downregulated by the tumour.
In contrast to the prophylactic vaccination against infectious disease or cancers associated with viral infection (cervical cancer, hepatocellular carcinoma), for cancer patients the only relevant vaccination strategy must be therapeutic. Generally speaking, cancer vaccines incorporate a source of tumour antigens combined with some type of “adjuvant” to make these tumour antigens visible to the immune system. Sources of tumour-associated antigens include whole autologous or allogeneic tumour cells, defined proteins, or specific peptide epitopes (see Figure 1). Most likely due to the heterogeneous histology of lung cancers, the relevant immunologically dominant antigens remain unknown. Therefore, the use of autologous tumour cells might be especially suitable for vaccination strategies in lung cancer, because no prior knowledge of specific tumour antigens is necessary and the induced immunity may not be confined to a single, specific antigen that could be downregulated by the tumour.
GVAX®
The genetic modification of autologous tumour cells to secrete immunomodulatory cytokines has been shown to induce antitumour immunity in a number of preclinical models. Of these cytokines, GM-CSF has demonstrated the greatest induction of antitumour immunity25. Two early-phase clinical trials using GM-CSF-secreting, autologous tumour cells (GVAX®) in patients with NSCLC have revealed encouraging preliminary results. Salgia and coworkers reported on safety and feasibility of this approach in 33 advanced NSCLC patients with the most common toxicities being local injection site reactions and flu-like symptoms. A mixed response in one patient and long recurrence-free intervals in two other patients following isolated metasectomy were observed26. In another phase I/II trial using the GVAX® platform, autologous tumour cells were transduced with GM-CSF through an adenoviral vector (Ad-GM) and administered as a vaccine27. 78% of patients developed antibody reactivity against allogeneic NSCLC cell lines. Three durable complete responses were observed.
MUC1 Vaccines
Mucin-1 (MUC1) is expressed on the cell surface of many common adenocarcinomas, including lung cancer. Because of its involvement in cell-cell interaction between malignant and endothelial cells, anti-MUC1 strategies may be useful in preventing metastatic spread of tumour cells in addition to their direct anti-tumour effect. A phase I study using a modified vaccinia virus (Ankara) expressing human MUC1, which also contains a coding sequence for human IL-2 (TG4010), revealed a safe toxicity profile and some clinical activity28. The phase II trial is currently underway as a multicentre study.
MUC1 has also been targeted in another trial of patients with NSCLC using the vaccine L-BLP25 (Stimuvax®). A multicentre phase IIB study investigating the vaccine in NSCLC patients' stages IIIB and IV has recently been updated with promising results for safety and clinical effectiveness in the first publication29,30. All patients had shown stable disease or a clinical response following standard first-line chemotherapy and were then vaccinated with Stimuvax or received best supportive care alone. Although the overall survival did not reach statistical significance, the survival in patients with stage IIIB (locoregional disease) improved at three years compared to stage IIIB patients with malignant pleural effusion and stage IV patients with 48.6% and 26.7%, respectively. An international, randomised, multicentre phase III trial for unresectable stage III NSCLC patients with stable disease or better following first-line chemoradiation has been initiated and the first patient has entered the trial in the US.
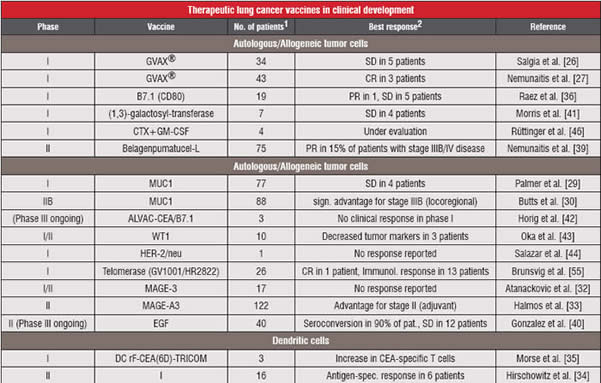
Table 2 : Lists Therapeutic Lung Cancer Vaccines Currently in Clinical Development.
Recombinant MAGE-A3 Protein Vaccine
Another protein vaccination strategy aims at the melanoma-associated antigen E-3 (MAGE-3), which is expressed in about 30% - 50% of lung cancers depending on stage and histological subtype and may be associated with poor prognosis31. First results reporting the successful induction of humeral and cellular immune responses in patients with NSCLC following vaccination with MAGE-3 with and without adjuvant chemotherapy have been published in 200432. Seventeen patients were enrolled following surgical resection with no evidence of disease. Nine patients received 300µg of the MAGE-3 protein alone, whereas 8 patients were treated with MAGE-3 combined with the adjuvant AS02B. In the first cohort (no adjuvant), only one patient showed a CD4+ T cell response. In contrast, 4 patients out of the second cohort (MAGE-3 plus adjuvant) developed a CD4+ T cell responses against the MAGE-3.DP4-peptide. Based on these results, a multinational phase II trial investigating the therapeutic efficacy of the MAGE-3 vaccine in patients with resected MAGE-3-positive stage IB/II NSCLC was initiated and recently completed. In this placebo-controlled study, 122 early-stage patients (MAGE-3-positive NSCLC) where vaccinated five times at three-week intervals. Preliminary analyses presented at the 2006 ASCO meeting33 revealed a 33% disease-free survival improvement for the resected and vaccinated patients. No significant toxicities were observed. A large multicentre phase III study is currently being initiated based on these results.
Other Vaccination Strategies
More lung cancer vaccines are currently being tested37,38, but review of all strategies in clinical development would go beyond the scope.
Summary and Conclusion
For long, lung cancer was not considered an immune-sensitive malignancy. With insufficient knowledge of relevant tumour antigens, lung cancer immunotherapy lags behind similar efforts in melanoma, renal cell and prostate cancer. However, there is increasing evidence that NSCLC and SCLC can evoke specific humeral and cellular anti-tumour immune responses. With increasing knowledge about the link between the induced immune response and a resulting objective clinical response, targeted agents may hold great promise in sequence with other (adjuvant) anti-tumour therapeutics. Obviously, not all of the immunotherapeutic approaches in the treatment of lung cancer can be mentioned in this review, e.g. adoptive cell transfer, immuno-gene therapy, inducers of apoptosis, certain signal transduction inhibitors and others47-50 have not found their way into later clinical development and, therefore, weren’t the focus of this review.
Acknowledgement
This work was supported by the Chiles Foundation, Portland, Oregon, USA, and the Walter-Schulz-Foundation, Munich, Germany.
References
1. Thun MJ, Henley J, Burns D et al.: Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst 2006;98:691-699.
2. Krasna MJ, Reed CE, Nugent WC et al.: Lung cancer staging and treatment in multidisciplinary trials: Cancer and Leukemia Group B cooperative group approach. Thoracic surgeons of CALGB. Ann Thorac Surg 1999;68:201-207.
3. Gura T: Magic bullets hit the target. Nature 2002;417:584-586.
4. Egri G, Takats A: Monoclonal antibodies in the treatment of lung cancer. Eur J Surg Oncol 2006;32:385-394.
5. Reissmann PT, Koga H, Figlin RA et al.: Amplification and overexpression of the cyclin D1 and epidermal growth factor receptor genes in non-small cell lung cancer. Lung Cancer Study Group. J Cancer Res Clin Oncol 1999;125:61-70.
6. Kim ES, Mauer AM, Tran HT et al.: A phase II study of cetuximab, an epidermal growth factor receptor (EGFR) blocking antibody, in combination with docetaxel in chemotherapy refractory/resistant patients with advanced non-small cell lung cancer: final report. Proc Am Soc Clin Oncol 2003;22:642a.
7. Hanna N, Lilenbaum R, Ansari R et al.: Phase II trial of cetuximab in patients with previously treated non-small-cell lung cancer. J Clin Oncol 2006;24:5253-5258.
8. Robert F, Blumenschein G, Herbst RS et al.: Phase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naïve advanced non-small-cell lung cancer. J Clin Oncol 2005;23:9089-9096.
9. Jensen AD, Munter MW, Bischoff H et al.: Treatment of non-small cell lung cancer with intensity-modulated radiation therapy in combination with cetuximab: the NEAR protocol (NCT00115518). BMC Cancer 2006;6:122.
10. Perez-Soler R, Saltz L: Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol 2005;23:5235-5246.
11. Heymach JV, Nilsson M, Blumenschein G et al.: Epidermal growth factor receptor inhibitors in development for the treatment on non-small cell lung cancer. Clin Cancer Res 2006;12:4441s-4445s.
12. Gatzemeier U, Groth G, Butts C et al.: Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small cell lung cancer. Ann Oncol 2004;15:19-27.
13. Clamon G, Herndon J, Kern J et al.: Lack of trastuzumab activity in non-small cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer 2005;103:1670-1675.
14. Langer CJ, Stephenson P, Thor A et al.: Eastern Cooperative Oncology Group Study 2598: trastuzumab in the treatment of advanced non-small-cell lung cancer: is there a role? Focus on Eastern Cooperative Oncology Group Study 2598. J Clin Oncol 2004;22:1180-1187.
15. Finzel AH, Reininger AJ, Bode PA et al.: ICAM-1 supports adhesion of human small cell lung carcinoma to endothelial cells. Clin Exp Metastasis 2004;21:185-189.
16. Johnson DH, Fehrenbacher L, Novotny WF et al.: Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-2191.
17. Sandler AB, Johnson DH, Herbst RS: Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer. Clin Cancer Res 2004;10:4258s-4262s.
18. Herbst RS, Johnson DH, Mininberg E et al.: Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol 2005;23:2544-2555.
19. Sandler A, Gray R, Perry MC et al.: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-2550.
20. Krug LM: Vaccine therapy for small cell lung cancer. Semin Oncol 2004;31:112-116.
21. Giaccone G, Debruyne C, Felip E et al.: Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B;Silva Study). J Clin Oncol 2005;23:6854-6864.
22. Elias DJ, Hirschowitz L, Kline LE et al.: Phase I clinical comparative study of monoclonal antibody KS1/4 and KS1/4-methotrexate immunoconjugate in patients with non-small cell lung carcinoma. Cancer Res 1990;50:4154-4159.
23. Lynch TJ Jr, Lambert JM, Coral F et al.: Immunotoxin therapy of small-cell lung cancer: a phase I study of N901-blocked ricin. J Clin Oncol 1997;15:723-734.
24. Ross HJ, Hart LL, Swanson PM et al.: A randomized, multicenter study to determine the safety and efficacy of the immunoconjugate SGN-15 plus docetaxel for the treatment of non-small cell lung carcinoma. Lung Cancer 2006;54:69-77.
25. Dranoff G, Jaffee E, Lazenbay A: Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 1993;90:3539-3543.
26. Salgia R, Lynch T, Skarin A et al.: Vaccination with irradiated autologous tumor cells engineered to secrete Granulocyte-Macrophage Colony Stimulating Factor augments anti-tumor immunity in patients with metastatic non-small cell lung carcinoma. J Clin Oncol 2003;21:624-630.
27. Nemunaitis J, Sterman D, Jablons D et al.: Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst 2004;96:326-331.
28. Rochlitz C, Figlin R, Squiban P et al.: Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med 2003;5:690-699.
29. Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A, Kehoe M, MacLean G, Longenecker M: Phase I study of the BLP25 (MUC1 Peptide) liposomale vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer 2001;3:49-57.
30. Butts C, Murray N, Maksymiuk A et al.: Randomized Phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 2005;23:6674-6681.
31. Gure AO, Chua R, Williamson B et al.: Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res 2005;11:8055-8062.
32. Atanackovic D, Altorki NK, Stockert E et al.: Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol 2004;172:3289-3296.
33. Halmos BH: Lung cancer II. ASCO Annual Meeting Summaries 2006;156-160.
34. Hirschowitz EA, Foody T, Kryscio R et al.: Autologous dendritic cell vaccines for non-small cell lung cancer. J Clin Oncol 2004;22:2808-2815.
35. Morse MA, Clay TM, Hobeika AC et al.: Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res 2005;11:3017-3024.
36. Raez LE, Cassileth PA, Schlesselmann JJ et al.: Allogeneic vaccination with a B7.1 HLA-A gene-modified adenocarcinoma cell line in patients with advanced non-small-cell lung cancer. J Clin Oncol 2004;22:2800-2807.
37. Raez LE, Rosenblatt JD, Podack ER: Present and future of lung cancer vaccines. Expert Opin Emerg Drugs 2006;11:445-459.
38. Nemunaitis J, Nemunaitis J: A review of vaccine clinical trials for non-small cell lung cancer. Expert Opin Biol Ther 2007;7:89-102.
39. Nemunaitis J, Dillman RO, Schwarzenberger PO et al.: Phase II study of LucanixTM (belagenpumatucel) a transforming growth factor 2 (TGF-beta 2) antisense gene modified allogeneic tumour cell vaccine in non small cell lung cancer (NSCLC). J Clin Oncol 2006;24:4721-4730.
40. Gonzalez G, Crombet T, Catala M et al.: A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: report of a pilot clinical trial. Ann Oncol 1998;9:431-435.
41. Morris JC, Vahanian N, Janik JE et al.: Phase I study of an antitumor vaccination using a-(1,3) galactosyltransferase expressing allogeneic tumor cells in patients with refractory or recurrent non-small cell lung cancer (NSCLC). J Clin Oncol 2005;23(16S):187s (abstract 2586).
42. Horig H, Lee DS, Conkright W et al.: Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother 2000;49:504-514.
43. Oka Y, Tsuboi A, Taguchi T et al.: Induction of WT1 (Wilm’s tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA 2004;101:13885-13890.
44. Salazar LG, Fikes J, Southwood S et al.: Immunization of cancer patients with Her-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res 2003;9:5559-5565.
45. Brunsvig PF, Aamdal S, Gjertsen MK et al.: Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother 2006;55:1553-1564.
46. Rüttinger D, van den Engel NK, Winter H et al.: Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical results and evidence of an immune response. J Transl Med 2007;5:43.
47. Hege KM, Carbone DP: Lung cancer vaccines and gene therapy. Lung Cancer 2003;41:S103-S113.
48. Maione P, Rossi A, Airoma G et al.: The role of targeted therapy in non-small cell lung cancer. Crit Rev Oncol 2004;51:29-44.
49. Rossi A, Maione P, Colantuoni G et al.: The role of new targeted therapies in small-cell lung cancer. Crit Rev Oncol 2004;51:45-53.
50. Rüttinger D, Winter H, van den Engel NK et al.: Immunotherapy of lung cancer: An update. Onkologie 2006;29:33-38.