
Combination treatment with multiple anti-cancer agents can be more effective compared to individual treatment. Thus, the synergistic interactions of a compound from the ginger family, 1’-S-1’-acetoxychavicol acetate, with Mycobacterium indicus pranii and cisplatin were investigated using mouse model, which showed promising results for further development to control breast cancer progression.
The use of compounds derived from natural sources has proven to be highly effective in the development of anti-cancer agents. One of such compounds extracted from the Malaysian ginger Alpinia conchigera, 1’-S-1'-Acetoxychavicol Acetate (ACA), has been repeatedly shown to suppress malignant characteristics and induce cell death in various types of cancer. In our previous studies, ACA was found to confer anti-cancer effects through the inhibition of the NFκB regulated proteins (In et al., 2012). The NFκB signalling pathway has been frequently implicated in cancer development, the upregulation of which confers a pro-survival advantage for cancer cells by affecting various cellular processes such as proliferation, angiogenesis and inflammation (Xia et al., 2014). Further investigations in the same study also revealed that ACA potentiates the effects of the FDA-approved anti-cancer medication, cisplatin (CDDP), thus highlighting the potential of using ACA in combination treatment.
Combination treatment, when carried out at optimum dose,is a useful alternative to deal with weaknesses of individual cancer treatment, either by amplifying the anti-cancer effects or by targeting different aspects of cancer phenotypes. Furthermore, anti-cancer drugsin clinical uses are often hampered by reduced efficacy in patients due to drug resistance despite exhibiting potent anti-cancer effects in pre-clinical settings.For example, CDDP is a commonly used chemotherapeutic drug that induces cytotoxicity in the rapidly dividing cancer cells by inhibiting DNA replication. However, patients are shown to develop resistance towards CDDP after the initial treatment (Shen et al., 2012), due to various reasons such as reduced cellular uptake of the compound or increased expression of oncogenic proteins such as NFκB. As such, treatment with more than one anti-cancer drug has shown promising results in overcoming the low efficacy of a compound and drug resistance. In the case ofCDDP resistance, inactivation of NFκB by pre-treatment with genistein, a soy-derived natural compound, was shown to increase the efficacy of cisplatin compared to treatment with cisplatin on its own (Li et al., 2005).
In testing ways to further augment the anti-cancer effects of ACA in vivo, we combined ACA treatment with cisplatin (CDDP)and Mycobacterium indicus pranii (MIP) in breast cancer (Subramaniam et al., 2019).MIP is a saprophytic non-pathogenic bacterium with immunotherapeutic potential.As it shares structural similarity with the leprosy bacterium, it is also effective in activating the immune system to treat bacterial diseases. In fact, MIP is an approved vaccine for leprosy that has also shown positive results against other diseases such as tuberculosis, indicating its effectiveness in stimulating the immune response (Singh et al., 2017; Duthie et al., 2018).In our preliminary analysis of MIP cytotoxicity, we discovered that heat-killed fraction of MIP was able to selectively induce apoptotic cell death in cancer cells with minimal effects on normal cells (Subramaniam et al., 2016). Furthermore, MIP has also been shown to improve the anti-cancer response when used as an adjuvant treatment (Ahmad et al., 2011). Specifically, MIP’s role in eliciting the immune response was demonstrated to occur through the activation of CD4+ T helper and CD8+ cytotoxic T cells (Rakshit et al., 2012).
In one of our previous studies, in vitro combination treatment with ACA, MIP and CDDP showed inactivation of NFκB and induction of apoptotic cell death in breast cancer (Subramaniam et al., 2018). As such, an in vivo study was carried outto further validate the synergistic effect of combining the cytotoxicity of ACA and CDDP with the immune-potentiating effect of MIP.The objectives of this study were to evaluate the effectiveness of double and triple combination therapies and investigate the effects of the treatments on NFκB and its regulated genes. Since MIP was a part of the combination treatment, the involvement of the immune response upon treatments was also analysed. As such, the BALB/c mice model was used as it is a strain useful for studying immune reaction and has been previously used for MIP related studies.The cell line used in this study is 4T1, a highly metastatic mouse breast cancer cell line that is capable of spontaneous metastasis into secondary tumour sites from the primary tumour, such as lymph nodes, blood, liver, lung, brain and bone.The use of a strain-specific murine cancer cell line instead of a human cell line was necessary to enable the subcutaneous tumour induction in BALB/c mice. Regardless of the origin, 4T1, derived from a stage IV mice breast cancer, is commonly used as a breast cancer model due to its similarity to human breast cancer (Pulaski & Ostrand-Rosenberg, 2001).
Once the tumour was established in the mammary pad region of the mice, treatments were administered subcutaneously at the tumour site twice a week for a duration of five weeks. To ensure optimum and safe treatment, the dosage for standalone treatments was based on the previous in vivo studies, while the dosage for combination treatments was based on thein vitro study which showed synergistic effect (In et al., 2012; Rakshit et al., 2012; Arshad et al., 2015; Subramaniam et al., 2018). The tumour volume and mice body weight were monitored and measured throughout the study, and histopathological analysis was carried out on the major organs harvested upon sacrifice at the end of the study.
For the duration of the study, mice from all treatment groups,which are the standalone treatment with ACA, MIP and CDDP, double combination treatment with ACA/MIP and ACA/CDDP and triple combination treatment with ACA/MIP/CDDP, were measured to be between 15-20 g of body weight. In terms of tumour regression, all treatment groups showed significant tumour volume reduction after five weeks of treatment compared to the placebo control. Of these groups, the highest reduction was seen in the triple combination treatment group. Furthermore, double combination of ACA/MIP exhibited the lowest tumour reduction compared to all the other treatment groups, suggesting the importance of CDDP mechanism of action in the synergistic effect during triple combination treatment.Meanwhile, analysis of major organs revealed significantly reduced metastasis in the triple combination treatment group, in which metastases were only observed in the lung with the foci of the lowest number and smallest area. Furthermore, examination of the metastases showed 100% necrotic cell damage, indicating the effectiveness of triple combination treatment in reducing metastasis and inducing tumour necrosis.
Next, blood obtained from the mice at the beginning and end of the study was used to analyse the changes in the expression of protein biomarkers and cytokines regulated by the NFκB signalling pathway. Cytokines, which are small proteins that play an important role in cell signalling, mediate a variety of cellular processes such as immune response and inflammation.The analysis was done on biomarkers associated with the onset of metastasis (VEGF and MMP-9), proteins regulating cell division (p300 and p21) and cytokines regulating inflammation and cancer progression (IL-2, IL-6,IL-10, IL-12, TNF-α and IFN-γ).
VEGF is signalling protein that stimulates blood vessel formation while MMP-9 is an enzyme that degrades the extracellular proteins. Both of these proteins are vital for the restructuring of the tumour environment during tumour development and progression. Based on the expression analysis, it was found that VEGF and MMP-9 were significantly downregulated during triple combination therapy. On the other hand, p300 and p21 are involved in cell maturation and cell-cycle, respectively. Their expression was significantly upregulated in the triple combination treatment group, which is the desired outcome when restricting tumour growth. As for the cytokines, the expression IL-10, which inhibits NFκB, was upregulated while pro-inflammatory cytokines IL-6,IL-12, TNF-α and IFN-γ were downregulated, indicating the inactivation of the NFκB pathway.
Overall, the examination of tumour development, histopathological analysis of major organs and blood serum analysis during treatment with ACA/MIP/CDDP provided conclusive evidence to support the synergistic anti-cancer effects of the triple combination treatment against breast cancer.
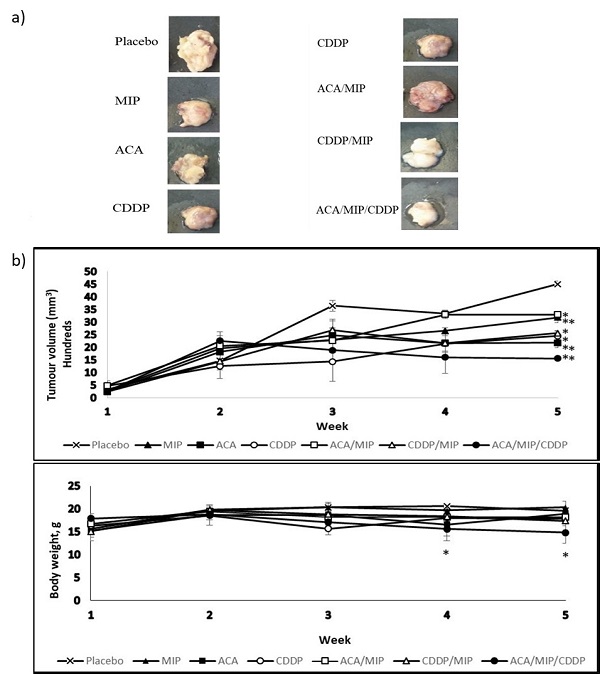
Figure 1.Tumour reduction effects of ACA combination treatments on BALB/c mice. (a) Images of tumours isolated from BALB/c mice 35 days post-treatment with a subcutaneous saline injection or with various 1'-S-1'-acetoxychavicol acetate (ACA), Mycobacterium indicus pranii (MIP), and cisplatin (CDDP) treatment regimens. All mice were terminated via euthanasia using a flow of pure carbon dioxide (CO2) in a gas chamber. Dissections were carried out and tumours were measured and fixed in a 10% (v/v) neutral buffered formalin (NBF) buffer solution for immunohistochemistry (IHC) analysis. (b) Tumour growth and body weight change in 4T1-bearing mice treated with different regimens over a period of five weeks. Tumour volume was calculated from the second week. Data were shown as mean ± standard deviation (SD). Statistically significant changes between the placebo and treatment groups on the fifth week are indicated as * for p< 0.05 and ** for p < 0.01. (reproduced from Subramaniam et al., 2019).
References
Ahmad, F., Mani, J., Kumar, P., Haridas, S., Upadhyay, P., & Bhaskar, S. (2011). Activation of anti-tumor immune response and reduction of regulatory T cells with Mycobacterium indicus pranii (MIP) therapy in tumor bearing mice. PLoS One, 6 (9), e25424.
Arshad, N. M., In, L. L., Soh, T. L., Azmi, M. N., Ibrahim, H., Awang, K., . . . Nagoor, N. H. (2015). Recombinant human alpha fetoprotein synergistically potentiates the anti-cancer effects of 1'-S-1'-acetoxychavicol acetate when used as a complex against human tumours harbouring AFP-receptors. Oncotarget, 6 (18), 16151-16167.
Duthie, M. S., Casper, C., & Reed, S. G. (2018). Second coming: the re-emergence and modernization of immunotherapy by vaccines as a component of leprosy control. Future Microbiol, 13, 1449-1451.
In, L. L., Arshad, N. M., Ibrahim, H., Azmi, M. N., Awang, K., & Nagoor, N. H. (2012). 1'-Acetoxychavicol acetate inhibits growth of human oral carcinoma xenograft in mice and potentiates cisplatin effect via proinflammatory microenvironment alterations. BMC Complement Altern Med, 12, 179.
Li, Y., Ahmed, F., Ali, S., Philip, P. A., Kucuk, O., & Sarkar, F. H. (2005). Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res, 65 (15), 6934-6942.
Pulaski, B. A., & Ostrand-Rosenberg, S. (2001). Mouse 4T1 breast tumor model. Curr Protoc Immunol, Chapter 20, Unit 20 22.
Rakshit, S., Ponnusamy, M., Papanna, S., Saha, B., Ahmed, A., & Nandi, D. (2012). Immunotherapeutic efficacy of Mycobacterium indicus pranii in eliciting anti-tumor T cell responses: critical roles of IFNgamma. Int J Cancer, 130 (4), 865-875.
Shen, D. W., Pouliot, L. M., Hall, M. D., & Gottesman, M. M. (2012). Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev, 64 (3), 706-721.
Singh, B., Saqib, M., Gupta, A., Kumar, P., & Bhaskar, S. (2017). Autophagy induction by Mycobacterium indicus pranii promotes Mycobacterium tuberculosis clearance from RAW 264.7 macrophages. PLoS One, 12 (12), e0189606.
Subramaniam, M., In, L. L. A., Kumar, A., Ahmed, N., & Nagoor, N. H. (2016). Cytotoxic and apoptotic effects of heat killed Mycobacterium indicus pranii (MIP) on various human cancer cell lines. Scientific Reports, 6.
Subramaniam, M., Liew, S. K., In, L., Awang, K., Ahmed, N., & Nagoor, N. H. (2018). Inactivation of nuclear factor kappaB by MIP-based drug combinations augments cell death of breast cancer cells. Drug Des Devel Ther, 12, 1053-1063.
Subramaniam, M., Arshad, N. M., Mun, K. S., Malagobadan, S., Awang, K., & Nagoor, N. H. (2019). Anti-Cancer Effects of Synergistic Drug–Bacterium Combinations on Induced Breast Cancer in BALB/c Mice. Biomolecules, 9 (10), 626.
Xia, Y., Shen, S., & Verma, I. M. (2014). NF-kappaB, an active player in human cancers. Cancer Immunol Res, 2 (9), 823-830.