Radiological imaging is capable of providing \'functional\' information for biomedical characterisation of disease beyond volumetric visualisation of structure with high spatial resolution. Improved technologies enable further transparency beyond imaging interlinked with important economical aspects, such as Six Sigma for improved and efficient patient care.
Healthcare providers in developed countries are facing the challenge of improved and efficient hospital management focussed on a bottom-up approach for patient flow and management. Patient management relies on best medical care right from the hospital entry point. This first contact and the subsequent hospital care are crucial and require expertise of high-level professionals and economic expertise. Today, imaging presents the opportunity for guiding the further diagnostic and therapeutic pathways and thus leads to dedicated and personalised medicine.
Computed Tomography (CT) is one of the leading imaging modality to visualise the structure of tumours. Novel volume acquisitions with almost isotropic voxels in sub-millimetres as provided by multislice CT enable a real volumetric approach. The large number of source images represents an incredible amount of work for the radiologists and is unacceptable for clinical partners. This requires the extraction of information from high-resolution volumetric
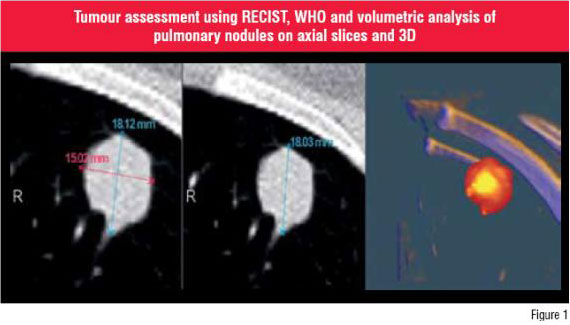
CT and has driven the development, implementation and integration of dedicated Computer Assisted Diagnosis (CAD) applications. They provide enhanced view of structure by multiplanar reformations, thin- or thick-slab maximum-intensity-projections, volume rendering, and virtual endoscopies. The sophisticated tools that have widespread application include detection and volumetry of pulmonary nodules (Figure 1) and virtual colonoscopy.
Both CT and Magnetic Resonance Imaging (MRI), provide whole body coverage and are useful to assess systemic, generalised diseases, e.g. M-staging in tumours and atherosclerosis.
Apart from extraction of information from a single series, steps such as fusion, matching and registration of images and extracted information from different modalities (PET, CT, MRI), e.g. colour-coded parameter maps, are necessary to exploit the potential of complementary image information.
MRI enables high spatial resolution and the detailed visualisation of structure with high spatial resolution and T1- or T2-weighting. The application of T1-, T2- proton- or diffusion-weighted sequences aims at the visualisation of special properties of the tissue of interest, such as fluid or cellularity.
Functional imaging, which relates mainly to volumetric imaging that generates a large number of images (Figure 2 and 3), is also called 4D imaging. Dedicated software and software engineering is required to extract the information, which is 'functional' in a broader sense. Time resolved series acquired during contrast applications enable morphological and functional insights of small and large vascular structures-so called magnetic resonance angiography (MRA)-and its territories and branches.
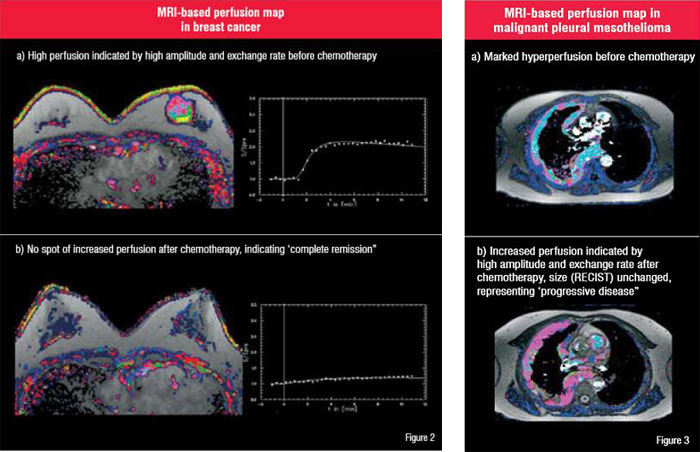
'Perfusion' MR imaging aims to capture tissue microcirculation by using endogeneous contrast or iatrogenic application of contrast agents (extra-cellular or intra-vascular) or even different contrast compartment mechanisms (one-, two- or multi-compartment model) on the basis of both T1-weighted or T2*-weighted sequences.
From such 4D-series (3D + time), perfusion is assessed qualitatively by visual evaluation, semi-quantitatively by analysing Signal Intensity (SI) or Signal to Noise Ratio (SNR) curves over time or by quantitative approaches that make use of more sophisticated pharmacokinetic modelling providing surrogates for microvessel density and vascular permeability. Both are regarded as indicators for the angiogenic potential of the tumour. Similar analyses are possible by contrast-enhanced CT (perfusion CT) that has entered clinical routine for stroke diagnostics and recently through the rapid improvement in CT-Scanner for cardiac imaging. Motion imaging is looking into cardiac and respiratory motion including the compliance of the myocardium (hypokinesia) and vascular walls. Appropriate triggering, e.g. by ECG or a respiratory belt is required. In aortic aneurysms the lack of compliance of the aortic wall might serve as a predictor of the risk of rupture. On other occasions, resolved image acquisition protocols allow for the assessment of respiratory motion and especially the mobility of neoplasms, which might have substantial effects on high precision radiotherapy planning.
Another novel functional MR technique is Diffusion Weighted Imaging (DWI), which provides additional insights into the microstructure of tissues simply by measuring the amount and direction of the diff sive Brownian motion of water molecules. It can be used for tractography (Diffusion Tensor Imaging or DTI) in the brain demonstrating the location and orientation of fibre tracts or visualization of recurrent cancer, especially in head and neck tumours, as it provides information on cellularity and perfusion.
Multidimensional imaging comprises targeted molecular imaging of individual tumour types and characteristics on the one hand and downstream surrogates of tumour biology, such as results from perfusion MR imaging, i.e. microcirculation and permeability that play a major role in oncological radiology on the other. The implementation of surrogate markers is a major challenge within the multidisciplinary concept of tumour diagnosis and treatment follow-up during dedicated therapies.
Once achieved, it will reduce the need for specific molecular imaging. Improved and increasingly sophisticated approach will integrate imaging and non-imaging surrogates, and will fuse different worlds within the life sciences. Future radiology will supply detailed structural and functional information, such as perfusion or metabolism as a 3D coverage of two different volumes
(1) 'whole body' volume for staging and (2) 'whole lesion' volume for assessment of heterogeneities.
In contrast to molecular biology, which mainly is unidimensional, and histology, which provides 2D information, radiology provides information regarding localization and extent in 3D. This situation demands for integration for such markers in large databases to enable a multidimensional matrix for prediction of prognosis or treatment response.
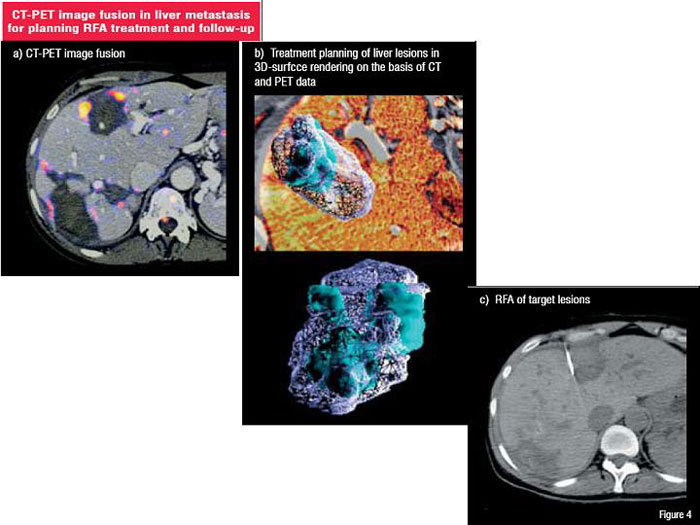
Continuous advances in therapeutic approaches necessitate new concepts in non-invasive image-based assessment. Nuclear medicine techniques, especially Positron Emission Tomography (PET), have recently gained importance with the combination of structural high resolution imaging from radiology (64-MSCT and higher) with a PET scanner (Figure 4).
In contrast, new functional imaging approaches such as dynamic contrast enhanced MRI (DCE-MRI) or Perfusion Computed Tomography (Perfusion CT) enable an additional tissue characterisation. These 4D (3D + t) high-resolution (spatial and temporal) imaging techniques are complementary to nuclear medicine. While PET enables assessment of metabolic activity 4D imaging using CT or MRI enables non-invasive insight of microvascular status in diagnosis of stroke or oncology. Perfusion imaging methodologies are of particular importance in the clinical setting, especially neo-adjuvant or adjuvant radio-chemotherapy in cancer including anti-angiogenesis. The combination of all these modalities enables a high level of individual patient care. The current transition to bio-medical imaging in radiology empowers medical professionals to respond more precisely to the individual disease status and predict the outcome.
Apart from volumetric structural visualisation with high spatial resolution CT and MRI, these modalities are capable of providing 'functional' information for individual characterisation of disease. Adding the dynamic temporal component to such acquisition allows generating 4D maps (3D structure + time). Two important tasks have to be solved by CAD (1) registration of different image series from different image modalities, and (2) extraction and integration of quantitative surrogates that can be used for characterisation, therapeutic decision-making, image-guided procedures and efficacy evaluation. This personalised medical value chain brings benefits to patient care from individual non-invasive 3D / 4D imaging-based surrogates and enables improvement of healthcare with innovative tools cost-effectively.
Today, healthcare providers in the developed countries are facing the heavy burden of healthcare costs, which are mainly caused by increased technological standards and the demographic challenges. At the same time, healthcare is one of the biggest and most promising industry worldwide and is experiencing a major change from public service to private patient-focussed care (so called: personalised medicine). Particularly, the major terms like 'individual patient care (diagnostics and therapy decision path)', 'process optimisation', 'market positioning' and 'Six Sigma' play a major role for department heads and hospital managers. In particular, Six Sigma is one of the important tools in healthcare management to rule out deficits in service quality and performance on different levels that affect the total patient clinical value chain. Therefore, we would like to make the reader familiar with the term: The term Six Sigma is derived from the electronic industry and often focusses on three important indicators in particular to healthcare providers - Access to services, Service cycle time, and Cycle time associated with result reporting and discharge. In Six Sigma, higher sigma numbers correspond to fewer defect rates. At the Six Sigma level, there are only 3.4 errors per million opportunities, which is nearly error-free. Striving towards the Six Sigma level involves five major steps:
1. Defining a problem
2. Measuring what is important
3. Using statistical tools to analyse the root causes for variation in quality and performance
4. Working together as a team for making improvements and implementing them over time
5. Monitoring and controlling the success and sustainability of the solutions.
Clearly defined measures and data analysis allow for additional benefits to manage the hospital processes effectively to increase individual patient care.