Left ventricular (LV) systolic function evaluation is based on ejection fraction assessment. Due to the great sensitivity of the examination, systolic time intervals (STI) are ideally suited to studying the effects of pharmacologic agents on the heart. By knowing the values of STI in ischemic heart disease (IHD) LV systolic dysfunction can be identified. Additionally, this method could have impending applications in the management of IHD.
Currently, cardiovascular diseases (CVD) is the leading cause of mortality in India. Ischemic heart disease (IHD) and stroke are the predominant causes and are liable for >80 per cent of CVD deaths. The quality of cardiovascular care in timely detection and variation in CVD treatment to halt the progression of the disease is of paramount importance. Systolic time intervals (STI) measurement provides a temporal representation of the stages of the cardiac cycle that occur sequentially and are physiologically impacted by factors such as heart rate, preload, afterload, and myocardial inotropic condition. Hence, the addition of easily available techniques like STI measurements in primary and secondary healthcare settings in India along with routine standard investigations is the need of the hour.
Additionally, it became clear from the literature review that there haven't been many studies in India that have used pulsed-Doppler echocardiography to assess STI in patients with IHD. As a result, our study aimed to assess STI in IHD patients and compare the results with those of healthy controls based on LV ejection fraction. (Fig 1).
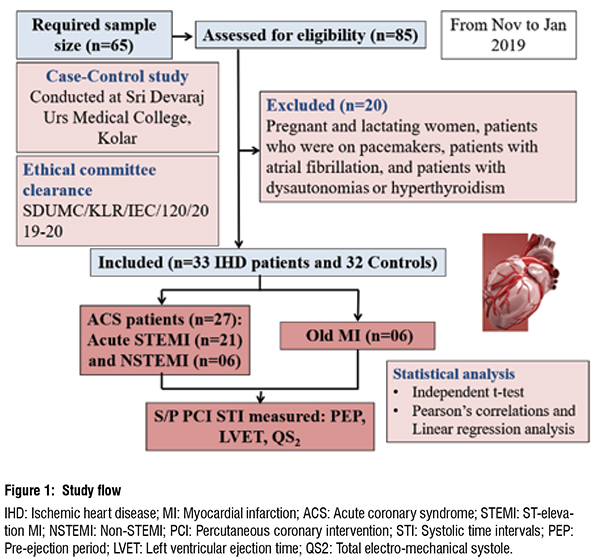
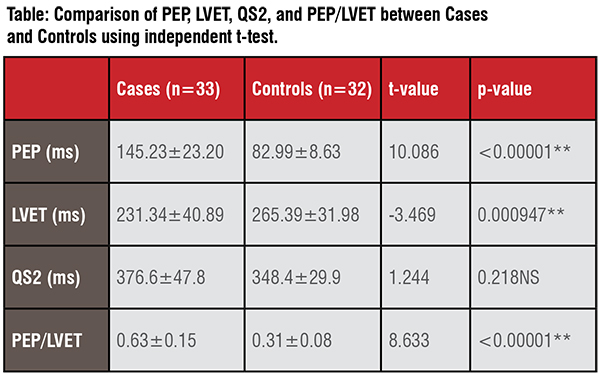
Our study results revealed a significant difference in STI between cases (n=33) and controls (n=32) (Table and Fig 2). Similar results were observed in a study conducted in France, in which PEP was found to be significantly prolonged; LVET was significantly reduced, with the subsequent significant rise in PEP/ LVET among heart failure (HF) patients using pulsed-Doppler echocardiography. In addition, they observed a correlation between LVEF and PEP/LVET (r = -0.55; P <0.001). Similar results were found in the current study (r = -0.368, P = 0.196) between LVEF ≤40per cent and PEP/ LVET about IHD patients.
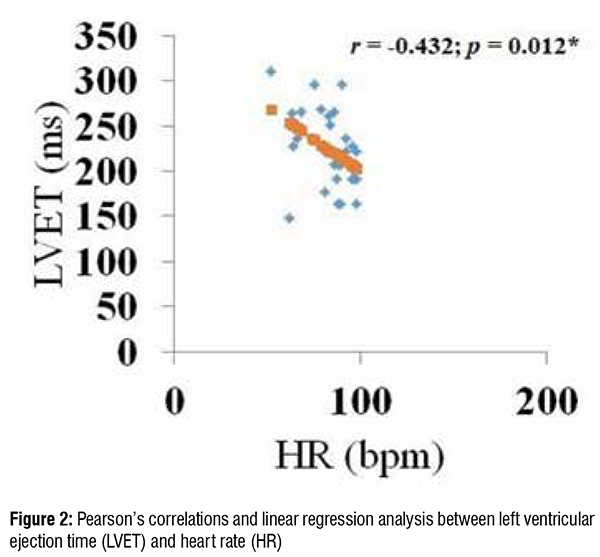
STI fluctuates inversely with heart rate (HR), thus corrections must be made, resulting in PEP index (PEPI), LVET index (LVETI), and QS2 index (QS2I). Even though these modifications are minor, they are substantial, and correcting the PEP for HR improves the method's sensitivity, particularly in clinical pharmacology. In this study, LVET and HR showed a rather strong association in the case of IHD patients (r=-0.432, P=0.012).
A prospective observational study by Sorrenson et al. included 1980 African-Americans of the Atherosclerosis Risk in Communities. Subjects underwent echocardiography and LVET was measured using pulsedwave Doppler. On multivariable adjustment for confounding variables, LVET continued to be an independent interpreter of incident HF. LVET additionally offered incremental prognostic data on the likelihood of future HF and death but not MI.
Besides, a normal STI among 32 IHD patients with mild to moderate mitral regurgitation was observed in the present study, which was in line with the literature.
Early acute myocardial infarction causes PEP, LVETI, and QS2I to shorten; late acute myocardial infarction causes prolonged PEP and short LVETI. Succeeding acute MI there will be a reduction in QS2I and ejection time (ET). Additionally, changes in PEP/LVET could be noticeable between the first and fourth days. Instead, the PEP will either be normal or lengthened. Twenty-seven acute MI patients were included in the current investigation (10 early and 17 late), and the STI also demonstrated comparable changes. It's possible that a longer isovolumetric contraction time contributed to the extended PEP. Known factors which reduce ET are tachycardia, reduced cardiac contractility, reduced stroke volume, catecholamines, digitalis, and isoprenaline administrations. The short-term effects of altered preload on STI were investigated in a different investigation by Khanna et al. in 17 AMI patients and 7 people without AMI. The effects of changing preload on STI were found to have the potential to give a more precise indicator of LV function in patients with AMI than the STI when assessed alone.
Excellent relationships between contractility markers and EF were found in a study by Parker et al. involving 36 male patients with electrocardiographically and angiographically apparent coronary artery disease (CAD). However, it was discovered that STIs were unreliable markers of ventricular function. Therefore, it is questionable whether or not STIs are useful in the clinical evaluation of CAD.
The median LV systolic ejection time (SET) was found to be shorter in heart failure patients with reduced ejection fraction (HFrEF) compared to those with preserved ejection fraction (HFpEF) (280 vs. 315 ms, P<0.001), the median PEP obtained was longer (114 vs. 89 ms, P<0.001), and the median relaxation time was shorter (78.7 vs. 93.3 ms In cases where there was a death or hospitalisation for HF, 11.8 per cent (n = 44) and 26.9 per cent (n = 46) of HFrEF patients respectively. Longer SET was independently linked with improved outcomes among HFrEF patients but not HFpEF patients, according to multivariate testing, indicating a potential role for stabilising SET as a beneficial strategy in patients with systolic dysfunction.
Therefore, utilising pulsed-Doppler echocardiography, our study was able to precisely assess systolic time intervals as an indication of LV systolic function in patients with IHD. STIs, particularly PEP/LVET, had strong correlations with traditional LV systolic performance indices such LVEF. Therefore, this approach may be particularly helpful for IHD patients with LVEF lower than 40 per cent. STI is a non-invasive, low-cost, and straightforward procedure that may be clinically effective for identifying individuals who have early LV dysfunction as well as for the treatment of IHD.
Pre-ejection period and PEP/LVET are linked to QRS width; henceforth corrections as described must be applied, which was not addressed in our study. Patients with congestive heart failure (NYHA class IV) or pulmonary edema will be in respiratory distress or are ventilated mechanically and thus the STIs cannot be obtained properly from them; accordingly, they were excluded from our study. Future prospective studies with large sample sizes need to be done to replicate and confirm our findings and the utility of this technique as routine investigation as a prognostic measure in IHD patients.
Declaration of patient consent: Written informed consent was obtained from all the study participants.
Funding: Self-funding.
Conflicts of interests: None declared.
References:
1. Prabhakaran, D., Jeemon, P., Roy, A. Cardiovascular Diseases in India Current Epidemiology and Future Directions. Circulation 2016;133:1605-1620. https://doi.org/10.1161/CIRCULATIONAHA.114.008729 .
2. Hassan, S., Turner, P. Systolic time intervals: a review of the method in the non-invasive investigation of cardiac function in health, disease and clinical pharmacology. Postgraduate Medical Journal 1983;59:423-434. https://doi.org/10.1136/pgmj.59.693.423 .
3. Seetharam SP, Shankar MS, Reddy N. Evaluation of systolic time intervals in patients of ischemic heart disease with clinical heart failure. J Pract Cardiovasc Sci 2022;8:84-9. https://doi.org/10.4103/jpcs.jpcs_17_22.
4. Reant, P., Dijos. M., Donal, E., Mignot, A., Ritter, P., Bordachar, P., et al. Systolic time intervals as simple echocardiographic parameters of left ventricular systolic performance: correlation with ejection fraction and longitudinal two-dimensional strain. European Journal of Echocardiography 2010;11(10):834–844. https://doi.org/10.1093/ejechocard/jeq084.
5. Boudoulas H. Systolic time intervals. European Heart Journal 1990 Dec;11 Suppl I:93-104. https://doi.org/10.1093/eurheartj/11.suppl_i.93.
6. Biering-Sørensen, T., Querejeta Roca, G., Hegde, S.M., Shah, A.M., Claggett, B., Mosley, T.H. Jr, et al. Left ventricular ejection time is an independent predictor of incident heart failure in a community-based cohort. Eur J Heart Fail 2018;20(7):1106-1114. https://doi.org/10.1002/ejhf.928.
7. Seetharam SP, Shankar MS, Reddy N. A narrative review of clinical applications of systolic time intervals. J Pract Cardiovasc Sci 2022;8:1-8. https://doi.org/10.4103/jpcs.jpcs_63_21.10.4103/jpcs.jpcs_63_21.
8. Weissler, A.M., Harris, W.S., Schoenfeld, C.D. Systolic time intervals in heart failure in man. Circulation 1968;37:149–59. https://doi.org/10.1161/01.CIR.37.2.149 .7
9. Khanna, P.K., Shah, P.M., Kramer, D.H., Schaefer, R.A., Tager, I. Effects of altered preload on left ventricular systolic time intervals in acute myocardial infarction. British Heart Journal 1973;35:1102-1108.
10. Parker, M.E., Just, H.G. Systolic time intervals in coronary artery disease as indices of left ventricular function: fact or fancy? British Heart Journal 1974;36:368-376.
11. Patel, P.A., Ambrosy, A.P., Phelan, M., Alenezi, F., Chiswell, K., Van Dyke, M.K . Association between systolic ejection time and outcomes in heart failure by ejection fraction. Eur J Heart Fail 2020;22(7):1174-1182. https://doi.org/10.1002/ejhf.1659.