Medical Equipment is a core asset for any healthcare facility. To ensure medical equipment is safe and effective there is a need to understand its associated management methodology. Consequently, a typical life cycle approach for medical equipment management is identified and explained in terms of processes and applications.
Medical equipment plays an important role in healthcare delivery. It ranges from small and simple devices such as sphygmomanometer to complex and big devices such as Magnetic Resonance Imaging (MRI) machines. This ranking is as a result of differences in utilised technologies and intended applications.
It is, therefore, of vital importance that healthcare organisations manage their assets to keep their expenditures under control as well as ensure the quality of healthcare delivery.
Medical Equipment Management (MEM) takes place within the context of human, material, structural, organisational, and financial resources. It is a process which helps hospitals to develop, monitor, and manage their equipment to promote the safe, effective, and economical use and maintenance of equipment. Responsible organisations should setup and regularly review MEM to ensure that a suitable medical device is used in accordance with the manufacturer’s instructions, maintained in a safe and reliable condition, and disposed appropriately at the end of its useful life.
A systematic way to manage medical equipment is to study and optimise all phases in the useful life of that equipment. A typical life cycle approach that was originally developed for major medical equipment, also applies to non-major but essential medical devices and may be extended to additional devices. It is a logical sequence of medical equipment management activities or stages, and each stage is dependent on and linked with other activities, as shown in Figure 1.
1. Planning
2. Acquisition
3. Delivery and incoming inspection
4. Inventory and documentation
5. Installation and commissioning
6. User training
7. Monitoring of performance
8. Maintenance
9. Replacement or disposal
Planning process is an important aid in decision-making because it provides essential information for management. In other words, it provides technology vision where healthcare facility should position itself; it can specify the following conditions in order to aid the decision-making process.
• Demonstrated needs and benefits
• Available qualified users
• Confirmed maintenance services and support
• Adequate environment support
• Regulatory compliance
These conditions are simple and should be applied to any routine acquisition of a medical device. A policy on medical device acquisition meeting these conditions as prerequisites to acquisition will reduce problems later in the life cycle of the device. For example, appropriate financial planning for a medical device can ensure optimum position for operating and service costs of this device.
Planning is the responsibility of the Medical Technology Advisory Committee (MTAC). The committee includes an administrator, a planning director, and a clinical engineering director. The role of planning is to ensure a balance between clinical and technology sectors of healthcare facilities in addition to meeting the community needs. The procedure of strategic planning of medical equipment includes:
• Performing an initial audit for existing technologies
• Conducting a technology assessment for new and emerging technologies that fit the desired clinical services
• Planning for replacement and selection of new technologies
• Setting priorities for acquisition
• Developing processes to implement equipment acquisition, and monitor ongoing utilisation
Healthcare industry is known for its continued innovation and production of new devices and techniques intended to improve the delivery and outcome of patient care. Funding constraint is considered the master key to evaluate incorporation of new technology to healthcare service. Thus, more attention should be given to the acquisition process keeping in mind both healthcare delivery outcomes and funding availability. Acquisition stage usually incorporates four main processes as shown in Figure 2.
Needs identification usually starts from users of technology, i.e. the medical staff (physicians and nurses).Indeed, the need to acquire a medical device may be due to one or a combination of the following reasons:
• Provide a new service
• Improve service efficiency
• Improve clinical outcomes
• Improve cost benefits
• Meet specific standards
• Reduce a risk.
In general, tendering process takes place to purchase medical equipment based on the required specifications. In tendering, all vendors are allowed to bid under a competitive and fair evaluation. Moreover, it gives a good opportunity for hospitals to select the best possible medical equipment. It is worthy to mention that technical specifications should include general requirements such as the warranty, technical services, technical documents, and any other necessary requirements for equipment operation.
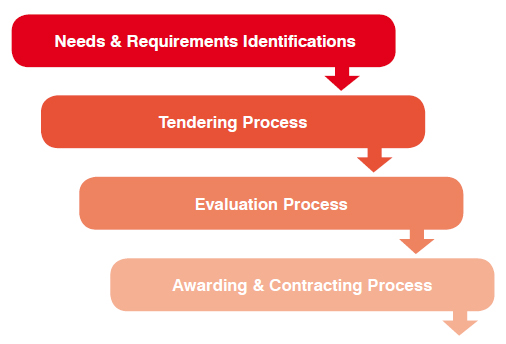
In the evaluation process, the purchased medical equipment should be evaluated from three different angles: technical, clinical, and financial. The purpose of technical and financial evaluations is to check the proposed technology, and to ensure the performance of the devices meets the desired outcomes. On the other hand, financial evaluation considers only the costs of the proposed technology. Both technical and clinical evaluations are carried out using either scoring or accept/reject approaches, whereas financial evaluation regards the lowest price among accepted vendors.
After making the selection, an award must be issued to acquire the device. A purchase contract document is prepared by the purchasing department and it must cover all terms and conditions that have been agreed upon by the vendor and the hospital.
Clinical engineering department ensures an incoming inspection on equipment includes verification of accessories, manuals, and electrical safety and operation in accordance with all applicable policies. Incoming equipment should be carefully checked for possible shipment damage and compliance with specifications in the purchase order. One role of clinical engineer is to ensure an incoming inspection on medical equipment by verifying the following:
• Accessories existence
• Manuals existence
• Electrical safety
• Compliance with specifications
• Possible shipment damage
Medical device inventory and documentation is an assistive stage in the life cycle. It provides information to support medical equipment management in different stages. Upon completion of the incoming inspection, a device record file should be created and it should be active throughout the useful life span of the device. Each device is identified and tracked by a unique number called equipment record number. The device record file should contain the following data:
• An Equipment Control Number (ECN)
• A generic description of the equipment
• The equipment manufacturer, model, and serial number
• The owner department and the location of the equipment
• The purchase order number and date
• The equipment’s acquisition cost
• The supplier’s name, address, and telephone number
• The warranty conditions and expiration date
• An abbreviated description of the inspection and preventive maintenance requirements and intervals
• An abbreviated service history
• Information regarding any applicable service contract
• The location of the equipment’s user and service manuals.
Installation and commissioning can be carried out by in-house technical staff if they are familiar with a given item of equipment. If the installation and commissioning are needed from the suppliers, in-house technical staff should monitor this process. In general, installation process should be compatible with standard policies for
medical equipment installation.
To reduce the possibility of equipment malfunction following service or repair, all personnel involved in maintaining and servicing equipment must be trained to appropriate standards for the work they are carrying out. Operator error is a leading cause of device malfunctioning, especially in developing countries. Incorrect usage of medical equipment will also greatly increase maintenance problems. Therefore, training of users should be regularly monitored from the vendor to ensure an appropriate skill level that is required for equipment operation. In fact, training should include all of the user staff as needed, such as clinical and technical staff. In addition, it should cover all aspects of medical equipment usage.
One common mistake in MEM is to believe that the warranty period is covered by the supplier, so no in-house technical attention is necessary. In-house technical staff should become the link between user and supplier and should observe any supplier's technical staff. This also will provide a learning opportunity for the in-house technical personnel. This performance should be also documented in the service history of the device by in-house technical staff.
Equipment maintenance involves all activities related to providing an adequate level of service and limiting downtime of medical devices. Maintenance or service activity is required in order to ensure the devices are kept functioning within the limits imposed by the test criteria and to return devices to the required level of functioning after breakage or other failure. The primary goal of maintenance activity is to reduce, or, if possible, to eliminate the need of repairs.
Traditionally, equipment maintenance is categorised as Preventive Maintenance (PM) and Corrective Maintenance (CM). Preventive maintenance procedures are actions that are necessary or desirable in order to extend the operational intervals between failures to extend the life of equipment or to detect and correct problems that are not apparent to the user. On the other hand, corrective maintenance procedures are any services that involve medical equipment repair, in addition to any specific service include repairs performed under a service contract or repairs performed by vendors during the warranty period.
It could be extended in case of a hazard notification or user error. In summary, PM aims keep the device as new as possible whereas CM aims to keep the device as good as prior to failure as possible.
Indeed, PM procedures are based on manufacturer's requirements, individual experience, and equipment service history, whereas CM procedures are mainly based on manufacturer's recommendations. Forward planning of maintenance calls for knowledge of maintenance requirements and the resources that are required in order to perform maintenance. These resources include labor, parts, materials, tools, and costs. PM should be performed based on the frequency and the procedure. Frequency of maintenance is based on the manufacturer’s recommendation and the equipment history. The maintenance procedure includes all
actions that should be carried out on a device. It should be written down for each device as a check list and reviewed regularly.
In CM, a response is carried out due to a service request. In this request, a summary of problem symptoms should be identified. Regardless of a technical service type, a set of factors can influence effectiveness of CM. These include experience, information, device complexity level, availability of spare parts, service manual existence, and equipped workshop. In this context, service modalities that provide CM are classified into four main classes; in-house service, contracted service, maintenance insurance, and contracted technology management. In-house service refers to maintaining the equipment by engineers and technicians in the clinical engineering department. The service contract is the most popular method for maintenance of medical equipment. Different options of this type are available based on labor and spare parts. In maintenance insurance, by its name, a hospital chooses to pay an insurance company instead of a service supplier. The insurance company then calls an appropriate service provider to support medical equipment. The last type is contracted technology management, in which all activities are completely assigned to management provider. In addition, a manager or a service provider is stationed in the hospital.
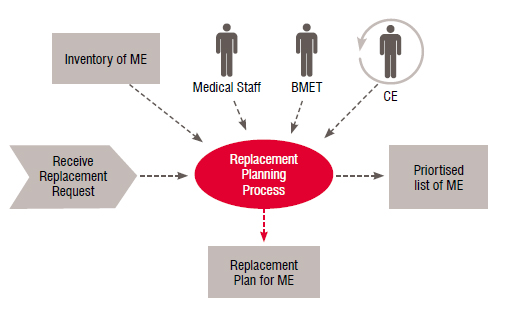
Replacement is the last stage of medical equipment's life cycle. All medical devices reach the point in their life where the cost-benefit ratio goes to the negative because of decreased reliability, increased downtime, safety issues, compromised care, increased operating costs, changing regulations, or simply obsolescence. A synopsis diagram that illustrates the replacement process in terms of participants, inputs, and output is shown in Figure 3.
Disposal of equipment must follow safety procedures in order to protect people and the environment. The ideal healthcare technology replacement planning system should be facilitywide, and cover all clinical equipment employing accurate objective data for analysis. Moreover, it should be futuristic and include strategic planning relating to clinical marketplace trends and the hospital’s strategic initiatives relating to technology. The plan should encompass factors relating to cost-benefit analysis, safety, expected life span, standardisation, and clinical benefits. In application, decontamination requirements should be regarded prior to disposal. Furthermore, many benefits can be obtained by utilising scrapped equipment as listed below:
• Use spare parts with similar equipment
• Replace with new ones with the same vendors
• Donate them to charity clinics after operation verification
• Dummies in internal training
• Use in research labs
• Save them for museums.
In fact, most of hospital planning processes tend to focus on current or short-term needs with little or no consideration of future replacement of medical equipment. An equipment replacement plan will help to guide the hospital on potential future spending obligations relating to medical devices. Different approaches are used for replacement of medical equipment. These approaches are either qualitative or quantitative. In qualitative approach, a combination of different criteria is regarded to approve replacement decision; whereas in quantitative approach, a mathematical model is proposed to determine replacement thresholds which lead to a realistic replacement decision.
REFERENCES
1. Dyro, J., “Clinical Engineering Handbook”, Elsevier Press, 2004.
2. Remmelzwaal, B. L., “The Effective Management of Medical Equipment in Developing Countries”, A Series of Five Papers, FAKT Project No. 390, Stuttgart, Germany, Jan. 1997.
3. MHRA, Department of Health, “Managing Medical Devices”, Medicines and Healthcare products Regulatory Agency Report, V1.0, United Kingdom, Apr. 2014.
4. Cameron, J. W., “Managing Medical Equipment in Public Hospitals”, Auditor General Victoria Report No. 9. State of Victoria, Australia, Mar., 2003.
5. Galbraith, E., “Electro-Medical Equipment Management”, NHS Fife, GP/E4, United Kingdom, version no1, Sept 2007.
6. Dickerson, M. L., and Jackson M. E., “Technology Management: A Perspective on System Support, Procurement and Replacement Planning”, Journal of clinical engineering, Mar-April, pp.129-136. 1992
7. Chan, A. Y. K., “Medical Technology Management Practice”, Charles C Thomas, USA, 2003.
8. Saleh, N., Sharawi, A., AbdElwahed, M., Petti, D., Puppato, D., and Balestra, G., “A New Approach for Preventive Maintenance Prioritization of Medical Equipment”, Proceedings of XIII Mediterranean Conference on Medical and Biological Engineering and Computing (MEDICON), Seville, Spain, Sep.2013, pp. 1059-1062.
9. Ouda, B. K., Mohamed, A.S., and Saleh, N., “A Simple Quantitative Replacement Model for Replacement of Medical Equipment Proposed for Developing Countries”, Proceedings of the 5th Cairo International Biomedical Engineering Conference, Cairo, Egypt, Dec. 2010, pp. 188-191.
10. Saleh, N., Rosati, S., Sharawi, A., Abdel Wahed, M., and Balestra, G., “Application of Quality Function Deployment and Genetic Algorithm for Replacement of Medical Equipment”, Proceedings of 7th Cairo International Biomedical Engineering Conference (CIBEC), Cairo, Egypt, Dec., 2014.