Currently, there are no approved anticancer drugs from Malaysia despite her abundance of natural resources. In efforts to identify natural compounds with the untapped potential to suppress and kill cancer cells, our lab has discovered various underlying mechanisms responsible for the anticancer properties of an active compound in Alpinia conchigera from the Zingiberaceae family, a perennial herb found locally in Malaysia. This active compound, 1'S-1'-acetoxychavicol acetate (ACA), has a terminal double bond and an acetoxyl group at position C-1’ which are known to be responsible for the cytotoxic or cell-killing effect against human cancers (Fig. 1). Preliminary studies using cell culture and animal model showed ACA induced apoptotic cell death and cell cycle arrest at the G0/G1 phase and suppressed migration in oral Squamous Cell Carcinoma (SCC) (Awang et al., 2010). In addition to the inhibiting growth of oral SCC, ACA was also found to further potentiate the effects of standard cisplatin (an FDA anticancer drug) treatment through modulation of the proinflammatory microenvironment (Phuah et al., 2013). These anticancer effects were due to ACA’s ability to inhibit the constitutive activation of an important transcription factor, NF-?B, by suppressing the activation of its kinase complex, IKK?/?. The Ubiquitin-Proteasome System (UPS) plays an important role in regulating cellular processes, and genes controlled by NF-?B associated with cancer survival have been found to be targeted by the proteasome. Through in silico docking study analysis, ACA was found to inhibit the proteasomal chymotrypsin-like activity of proteasome due to the interaction of their 1’-acetoxyl group with threonine active site of the proteasome (Liew et al., 2017).
The role of ACA in inducing autophagy, a regulated cell destructive and recycling mechanism, was also validated through a study on human non-small cell lung cancer (NSCLC). Autophagy plays a role in determining the fate of cells by inducing either survival or death. This study demonstrated inhibition of cancer cell viability by ACA through the formation of cytoplasmic vacuoles (Sok et al., 2017). Induction of autophagy was also shown after ACA treatment in NSCLC through the increased formation of acidic vesicular organelles and GFP-LC3 punctate formation, which are critical steps in cells undergoing autophagy. Further analysis revealed more evidence to verify the induction of autophagy by ACA, such as the accumulation of LC3-II and degradation of p62 upon exposure to ACA. Additionally, ACA was found to induce a Beclin-1-independent autophagy pathway, as ACA treatment was observed to cause a reduction in Beclin-1, an important regulator of autophagy and cell death. This was validated when autophagy was induced by ACA even when treated with an early autophagy inhibitor,3-methyladenine (3-MA). On the other hand, silencing the expression of LC3-II along with ACA treatment suppressed autophagy and induced apoptosis instead of NSCLC. Predictably, treating the cells with ACA and lysosomal inhibitor similarly resulted in apoptosis. Together, these results show that ACA induces a pro-survival autophagy in NSCLC, which takes place using a Beclin-1-independent pathway.
Once ACA’s anticancer potential was validated using both in vitro and in vivo models, it was necessary to assess the interaction between ACA and Cytochrome P450 (CYPs) superfamily metabolising enzymes. This is especially important if ACA is to be developed into a viable anticancer drug candidate, as the efficacy and toxicity of a drug may be compromised due to interactions with other drugs, which can affect the plasma levels of the drug by either increasing or decreasing it. As such, studies were carried out to profile the inhibitory potential and mechanism of inhibition and kinetics through fluorescent CYP inhibition assay. This study, which was done to evaluate ACA’s effect on nine human CYPs enzymes, found that ACA potentially interacts with co-administered drugs that are metabolized by several CYPs enzymes such as CYP1A2, CYP2D6, or CYP3A4 enzymes (Haque et al., 2017).
In looking at other aspects of ACA’s anticancer activity, the deregulation of microRNAs (miRNA) in cancer cells in response to ACA was also investigated. miRNAs are the short strand of RNAs that carry out post-transcriptional gene regulation by targeting and eliminating the expression of target mRNAs. The deregulation of miRNAs is well documented in most diseases, including various types of cancers. Generally, miRNAs that play oncogenic roles are found to be upregulated while miRNAs that play tumor suppressive roles are found to be downregulated in cancer cells. From the study which analyzed miRNA expression upon exposure to ACA, it was found that downregulation of miR-210 and miR-629 conferred sensitivity towards ACA by reducing cell proliferation and augmenting apoptosis in cervical cancer cells, by relieving the targeting of tumour suppressors SMAD4 and RSU1, respectively (Phuah et al., 2017a, 2017b). Results from this study highlight the possibility for a combination of miRNA-based therapeutics with ACA treatment to enable an increased efficacy in treating cervical cancer.
Although ACA demonstrates anticancer activity in vitro and in vivo, there are few issues impeding its development as an anticancer drug, which are poor solubility, depreciation of biological activity, and non-specific targeting of tumour cells. As such, several strategies to address these issues with ACA were explored. The first of such strategy was developing a novel conjugation between the recombinant Human Alpha-Fetoprotein (rhAFP) with ACA. Alpha-fetoprotein (AFP), a 70-kDa specific onco-embryonal protein, has been shown to have potential therapeutic value in treating various diseases such as autoimmune diseases and cancer (Dudich et al., 2012). Since rhAFP is a naturally occurring soluble human protein, the coupling of the non-soluble, easily hydrolysed ACA compound with rhAFP was able to enhance the in vivo solubility, create longer lasting activity and result in a more chemically stable drug for future commercialization (Arshad et al., 2015). Synergism in tumour cell killing by ACA-rhAFP was as a result of specific recognition of tumour cells that are known to express AFP-receptors. The increase in cell death may also be due to the ability of ACA to induce apoptosis via the extrinsic pathway effectively together with rhAFP that uses the intrinsic pathway. As a result of this combination effect, a lower effective dose of both drugs and a wider tumour genotype target was achieved. To validate the synergistic effect of ACA and rhAFP, athymic mice (without T cell-mediated immunity) with human prostate and lung cancer grafts were treated with stand-alone and combination regimens. The combination regimen resulted in a significant reduction of tumour volume when compared to the stand-alone regimen (Fig. 2), with lesser effects of systemic toxicity on body weight loss and vital organ inflammation (Fig. 3). The levels of tumour antigen marker were consistent for all of the treatment groups treated with ACA/rhAFP. On the other hand, levels of carcinoembryonic antigen (CEA) and prostate-specific antigen (PSA) were high at the beginning of the treatment and were reduced as the tumour bulk volumes decreased. Also, the combined treatments showed reduced levels of all important cancer-associated and inflammatory proteins.
Another strategy that was identified was the use of a triple combination of chemotherapeutic treatment, using the chemo-sensitising properties of ACA, the immuno-potentiating activity of a bacteria, Mycobacterium indicus pranii, and the cytotoxic properties of the commercially available FDA anticancer drug, cisplatin. The in vitro and in vivo models showed that the combined treatment displayed a higher level of killing and reduction in tumour volume when compared to the three stand-alone drug treatments (Subramaniam et al., 2016). As predicted, NF-?B and its regulated genes and inflammatory biomarkers were found to be downregulated during the combination treatment, as revealed by the immunohistochemistry analysis. The cytokine analysis showed evidence of immune activation, validating MIP’s ability to activate CD4+ T-helper cells. On a related note, an ongoing study carrying out acute and sub-chronic toxicity analyses of ACA in Sprague-Dawley (SD) rats found that the treatment of ACA for the 14-days acute study and 28-days sub-chronic are generally safe.
Overall, research on ACA has revealed the medicinal potential and chemo-sensitising properties of Malaysian phyto-compounds against cancer. Furthermore, the research has paved the way for ACA to be further developed clinically in order to be used in combination with FDA drugs, mycobacterium, and protein carrier for better therapeutic potential, pioneering the basis for the future combination of anticancer drug developments.
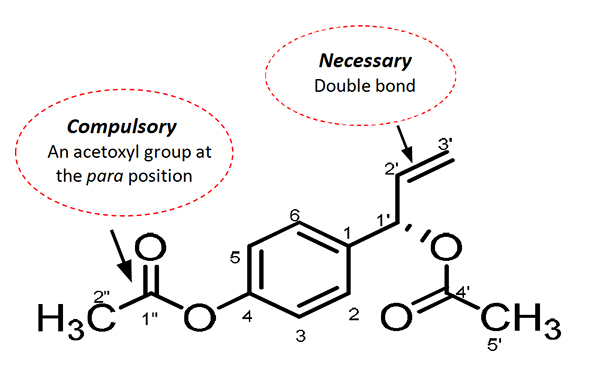
Figure 1: Chemical structure of the Malaysian isolate, ACA from Alpinia conchigera Griff. (Zingiberaceae) (adapted from Awang et al., 2010)
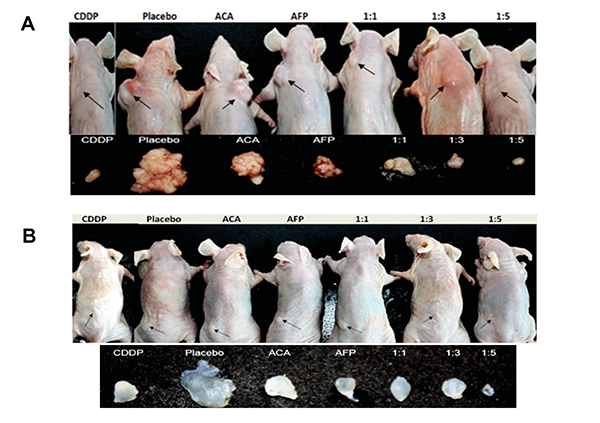
Figure 2: Tumour reduction effects of various rhAFP-ACA treatment regimens on athymic mice. Location of all surface tumour sites are indicated by closed arrows, and representative photographs (n=6) of tumours harvested 35 days post-implantation for (A) Human lung A549 tumour grafts and (B) Human prostate PC-3 tumour grafts. Saline solution 0.9 per cent (w/v) sodium chloride was used as placebo, while cisplatin (CDDP) (10.0 mg/kg) was used as a positive control reference. (adapted from Arshad et al., 2015)
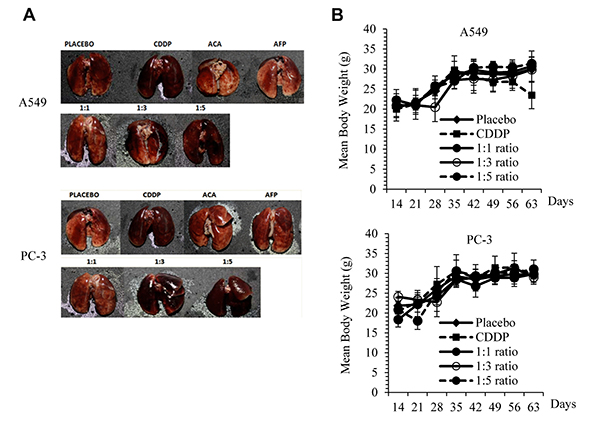
Figure 3: Physiological side effects of rhAFP-ACA in mice. (A) Signs of pulmonary inflammation and capillary haemorrhaging in cisplatin (CDDP) treated groups and at high rhAFP-ACA molar ratio regimens (?1:3) compared to placebo in A549 human lung and PC-3 human prostate tumour grafts. (B) Assessment on mean ± S.D. body weight loss between various combined rhAFP-ACA treatment groups of A549 lung and PC-3 prostate grafts. Placebo denotes groups treated with 0.9 per cent (w/v) sodium chloride solution while the concentration of CDDP was set at 10.0 mg/kg once per week. (adapted from Arshad et al., 2015)
References
Arshad, N. M., In, L. L., Soh, T. L., Azmi, M. N., Ibrahim, H., Awang, K., . . . Nagoor, N. H. (2015). Recombinant human alpha-fetoprotein synergistically potentiates the anti-cancer effects of 1'-S-1'-acetoxychavicol acetate when used as a complex against human tumours harbouring AFP-receptors. Oncotarget, 6(18), 16151-16167.
Awang, K., Azmi, M. N., Aun, L. I., Aziz, A. N., Ibrahim, H., & Nagoor, N. H. (2010). The apoptotic effect of 1's-1'-acetoxychavicol acetate from Alpinia conchigera on human cancer cells. Molecules, 15(11), 8048-8059.
Dudich, E., Dudich, I., Semenkova, L., Benevolensky, S., Morozkina, E., Marchenko, A., . . . Tatulov, E. (2012). Engineering of the Saccharomyces cerevisiae yeast strain with multiple chromosome-integrated genes of human alpha-fetoprotein and its high-yield secretory production, purification, structural and functional characterization. Protein Expression and Purification, 84(1), 94-107.
Haque, A., Leong, K. H., Lo, Y. L., Awang, K., & Nagoor, N. H. (2017). In vitro inhibitory mechanisms and molecular docking of 1'-S-1'-acetoxychavicol acetate on human cytochrome P450 enzymes. Phytomedicine, 31, 1-9.
Liew, S. K., Azmi, M. N., In, L., Awang, K., & Nagoor, N. H. (2017). Anti-proliferative, apoptotic induction, and anti-migration effects of hemi-synthetic 1'S-1'-acetoxychavicol acetate analogs on MDA-MB-231 breast cancer cells. Drug Design, Development and Therapy, 11, 2763-2776.
Phuah, N. H., Azmi, M. N., Awang, K., & Nagoor, N. H. (2017a). Down-Regulation of MicroRNA-210 Confers the Sensitivity towards 1'S-1'-Acetoxychavicol Acetate (ACA) in Cervical Cancer Cells by Targeting SMAD4. Molecules and Cells, 40(4), 291-298.
Phuah, N. H., Azmi, M. N., Awang, K., & Nagoor, N. H. (2017b). Suppression of microRNA-629 enhances sensitivity of cervical cancer cells to 1'S-1'-acetoxychavicol acetate via regulating RSU1. Oncotargets and Therapy, 10, 1695-1705.
Phuah, N. H., In, L. L., Azmi, M. N., Ibrahim, H., Awang, K., & Nagoor, N. H. (2013). Alterations of microRNA expression patterns in human cervical carcinoma cells (Ca Ski) toward 1'S-1'-acetoxychavicol acetate and cisplatin. Reproductive Sciences, 20(5), 567-578.
Sok, S. P., Arshad, N. M., Azmi, M. N., Awang, K., Ozpolat, B., & Hasima Nagoor, N. (2017). The apoptotic effect of 1'S-1'-Acetoxychavicol Acetate (ACA) enhanced by inhibition of non-canonical autophagy in human non-small cell lung cancer cells. PLoS One, 12(2), e0171329.
Subramaniam, M., In, L. L. A., Kumar, A., Ahmed, N., & Nagoor, N. H. (2016). Cytotoxic and apoptotic effects of heat killed Mycobacterium indicus pranii (MIP) on various human cancer cell lines. Scientific Reports, 6.
Subramaniam, M., Liew, S.K., In L. L. A., Awang K., Ahmed, N., & Nagoor, N.H. (2018). Inactivation of Nuclear Factor ?B by MIP based drug combinations augments cell death of breast cancer cells. Drug, Design, Development and Therapy.